| Product No. | CH050 |
|---|---|
| CAS Reg. No. | 1346602-97-8 |
| Alternate CAS Reg. No. | (unlabelled compound) |
| Offer | 5mg, 10 mg |
1346602-97-8
- Documentation
- Details
Chemical name
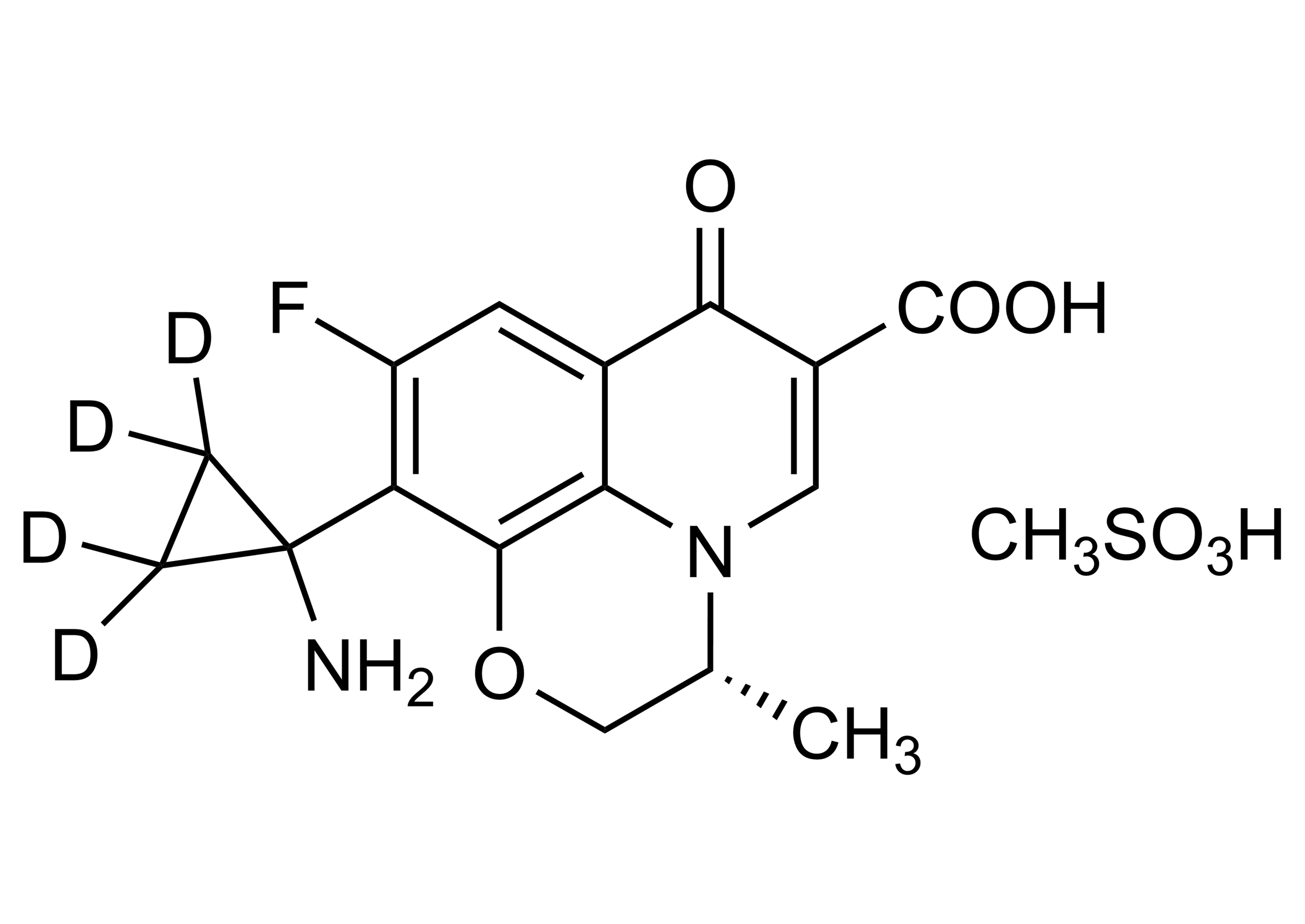
(3S)-10-(1-Aminocyclopropyl-D4)-9-fluoro-3-methyl-7-oxo-2,3-dihydro-7H pyrido[1,2,3-de]-1,4-benzoxazine-6-carboxylic acid methanesulfonate
Description
Pazufloxacin-D4 methanesulfonate Pazufloxacin-D4 mesylate (CAS 1346602-97-8) is a high-purity reference standard produced and distributed by WITEGA Laboratorien Berlin-Adlershof GmbH. It enables confident quantification in complex matrices and consistent reporting. It supports LC-MS/MS calibration, GC-MS confirmatory analysis, traceable quantification standard, method validation material, pharmaceutical metabolism research, multi residue screening, residue analysis reference, and regulated laboratory workflows.
This deuterium labeled salt provides precise isotope matching for accurate response ratios. Therefore it corrects for recovery and matrix effects during analysis. Use it to calibrate instruments, assess linearity, and verify selectivity. The reference standard includes lot specific purity and identity data with clear documentation. Furthermore, each batch maintains traceability that supports compliant audits.
For routine testing, it streamlines setup and reduces rework. Analysts prepare gravimetric stock solutions and working dilutions with confidence. LC-MS/MS and GC-MS methods benefit from stable signals and low background. The material suits residue control, metabolism studies, and research screening. Moreover, it supports confirmatory analysis when regulations require unambiguous results.
- High chemical purity and defined isotopic enrichment
- Traceable documentation and lot specific CoA
- Compatibility with LC-MS/MS and GC-MS workflows
- Effective for calibration, method validation, and system suitability
- Produced and distributed by WITEGA Laboratorien Berlin-Adlershof GmbH
- Regulated laboratories and quality control environments
- Pharmaceutical research and development
- Residue control and surveillance programs
- Multi residue method development across matrices
- Confirmatory analysis in compliance studies
Choose the Pazufloxacin-D4 methanesulfonate Pazufloxacin-D4 mesylate reference standard to strengthen data quality, calibration traceability, and method robustness across projects.
Safety Data Sheet
You can download your Safety Data Sheet for CH050
For other languages please contact us: witega@witega.de
Additional information
| Chemical name | (3S)-10-(1-Aminocyclopropyl-D4)-9-fluoro-3-methyl-7-oxo-2,3-dihydro-7H pyrido[1,2,3-de]-1,4-benzoxazine-6-carboxylic acid methanesulfonate |
|---|---|
| Molecular Formula | C16H11D4FN2O4 x CH3SO3H |
| Molecular Weight | 418.43 g/mol |
| Isotopic purity | > 99.0 atom% D (MS) |
| HPLC purity | > 99.0 % |
| Overall purity | > 99.0 % (HPLC) |
| Product Format | Neat |
| Delivery time | In stock |
| shelf life | 24 months |
| Storage | refrigerator, 2-8°C |
| Country of Origin | Germany |
| Product No. | CH050 |
| CAS Reg. No. | 1346602-97-8 |
| Alternate CAS Reg. No. | (unlabelled compound) |
| Offer | 5mg, 10 mg |