| Product No. | PS393 |
|---|---|
| CAS Reg. No. | 80060-09-9 |
| Alternate CAS Reg. No. | - |
| Offer | 250 mg |
80060-09-9
- Documentation
- Details
Chemical name
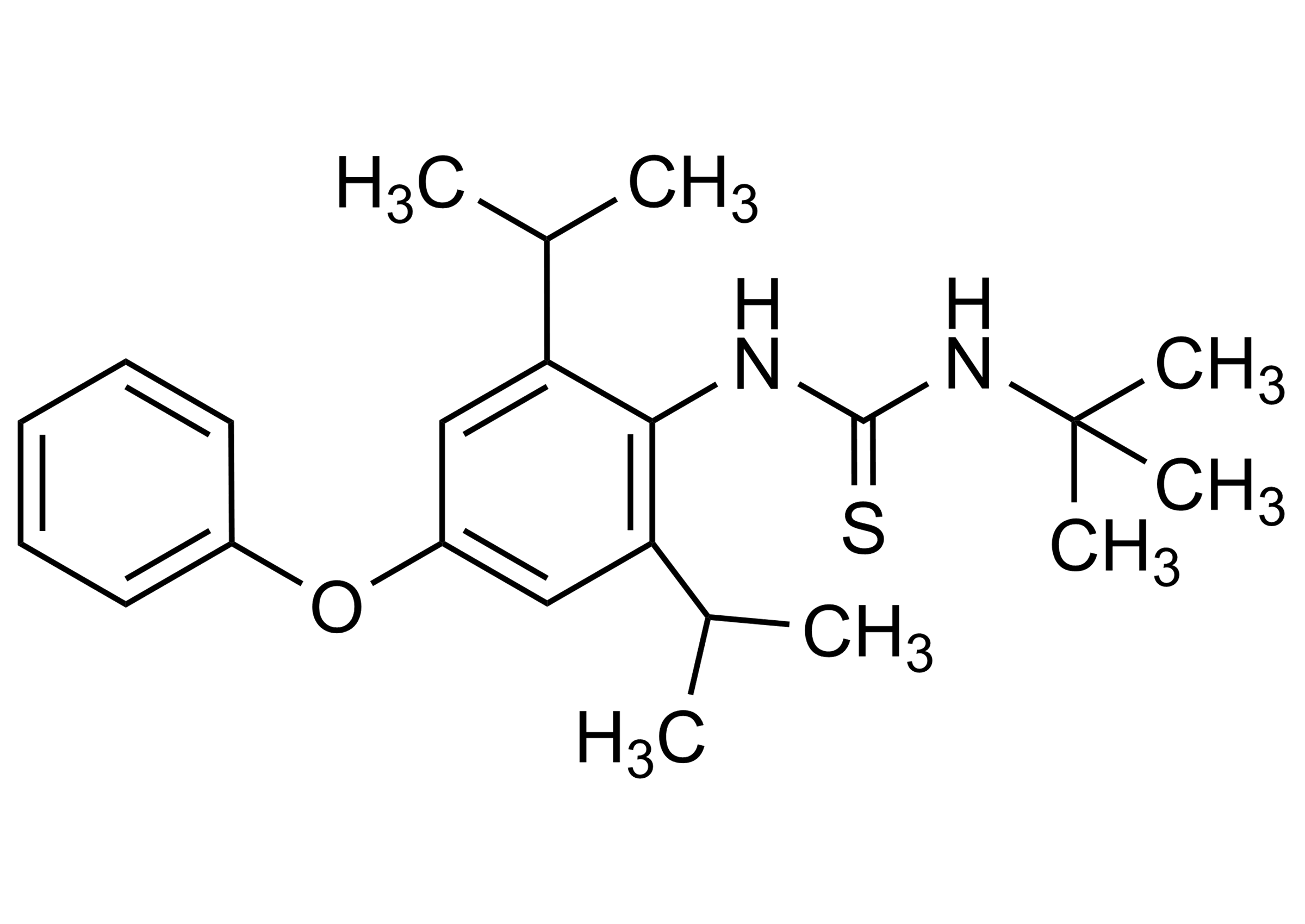
N-[2,6-Bis(1-methylethyl)-4-phenoxyphenyl]-N′-(1,1-dimethylethyl)thiourea
Description
Diafenthiuron (CAS 80060-09-9) reference standard from WITEGA Laboratorien Berlin-Adlershof GmbH supports LC-MS/MS and GC-MS workflows with dependable performance. As a pesticide residue analysis standard, it enables accurate calibration, traceable quantification, and confident confirmation across diverse matrices. Produced and distributed by WITEGA Laboratorien Berlin-Adlershof GmbH, it fits seamlessly into regulated laboratory routines and multi-residue screening.
Use this reference standard to establish calibration curves, verify instrument response, and confirm identity through ion ratios and retention time. Moreover, it assists in recovery checks, matrix effects assessment, and cross-platform comparability. Therefore, you can validate methods with clarity and maintain consistent measurement traceability over time.
Typical applications include:
- LC-MS/MS quantification and GC-MS confirmatory analysis
- Residue control in food, feed, and environmental samples
- Pharmaceutical and agrochemical research
- Metabolism studies and stability investigations
- Multi-residue method development and optimization
Key advantages:
- High-quality reference standard with batch-specific documentation for traceability
- Reliable purity and identity control for robust calibration
- Clear labeling to support method validation and quality control
- Consistent performance suited to routine and confirmatory analysis
Additionally, the material integrates into matrix-matched or solvent-based calibration schemes. It supports linearity checks, LOD and LOQ determination, precision assessments, and accuracy studies. Furthermore, laboratories can standardize results across sites using the same batch documentation, improving comparability and data integrity.
When setting up new protocols, use Diafenthiuron (CAS 80060-09-9) to bracket calibration ranges, evaluate carryover, and verify recovery in fortified samples. Also, apply it to monitor instrument performance trends and to confirm positives in screening workflows. This reference standard by WITEGA Laboratorien Berlin-Adlershof GmbH helps ensure reliable, traceable quantification from development to routine testing.
Safety Data Sheet
You can download your Safety Data Sheet for PS393
For other languages please contact us: witega@witega.de
Additional information
| Chemical name | N-[2,6-Bis(1-methylethyl)-4-phenoxyphenyl]-N′-(1,1-dimethylethyl)thiourea |
|---|---|
| Molecular Formula | C23H32N2OS |
| Molecular Weight | 384.58 g/mol |
| Isotopic purity | - |
| HPLC purity | > 98.0 % |
| Overall purity | > 98.0 % (HPLC) |
| Product Format | Neat |
| Delivery time | In stock |
| shelf life | 24 months |
| Storage | refrigerator, 2-8°C |
| Country of Origin | Germany |
| Product No. | PS393 |
| CAS Reg. No. | 80060-09-9 |
| Alternate CAS Reg. No. | – |
| Offer | 250 mg |