| Product No. | LA020 |
|---|---|
| CAS Reg. No. | not available |
| Alternate CAS Reg. No. | 26787-78-0 (unlabelled compound) |
| Offer | 5 mg, 10 mg |
not available
- Documentation
- Details
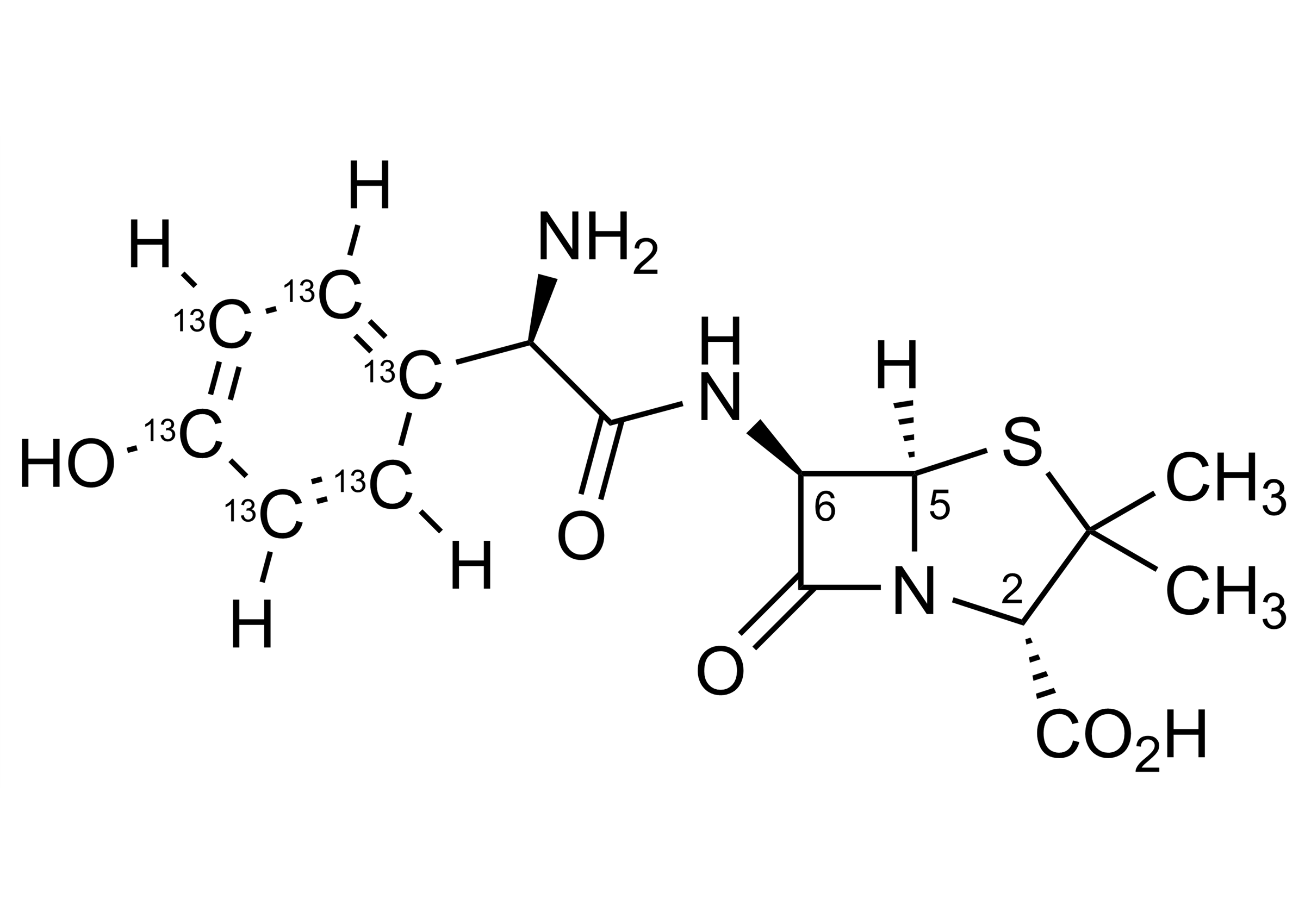
Chemical name
(2S,5R,6R)-6-[[(2R)-2-Amino-2-(4-hydroxyphenyl-13C6)acetyl]amino]-3,3-dimethyl-7-oxo-4-thia-1-azabicyclo[3.2.0]heptane-2-carboxylic acid
Description
Amoxicillin-13C6 is a stable isotope labeled reference standard produced and distributed by WITEGA Laboratorien Berlin-Adlershof GmbH. It supports LC-MS/MS and GC-MS quantification, calibration, method validation, and confirmatory analysis with excellent consistency. Therefore, laboratories can build robust calibration curves and ensure traceability across batches. Related searches: beta lactam antibiotic standard, LC MS MS calibration, GC MS confirmatory analysis, stable isotope labeled compound, pharmaceutical residue control, method validation material, traceable analytical reference, multi residue method development, metabolism studies, regulated laboratory workflows.
This reference standard helps achieve accurate analyte response alignment and reliable retention-time matching. Moreover, Amoxicillin-13C6 enables low-level quantification in complex matrices. It reduces matrix effects and supports verification of analyte identity. Furthermore, clear documentation and batch-specific data simplify audits and result reporting for regulated workflows.
Use this reference standard to strengthen method performance at every stage:
- Calibration in LC-MS/MS or GC-MS with matrix-matched or solvent-based curves
- Ongoing traceability and system suitability checks
- Confirmatory analysis with matched chromatographic behavior
- Method validation, including accuracy, precision, linearity, and recovery
Typical applications span across:
- Regulated laboratories requiring defensible results
- Pharmaceutical research and development programs
- Residue control and quality assurance in food and environmental testing
- Metabolism studies and kinetic profiling
- Multi-residue method development
Each Amoxicillin-13C6 reference standard from WITEGA Laboratorien Berlin-Adlershof GmbH is delivered with comprehensive documentation for traceability. Additionally, users benefit from controlled production, tight specifications, and proven stability. As a result, laboratories can implement routine workflows with confidence. Select Amoxicillin-13C6 to enhance comparability between runs, support accreditation goals, and maintain long-term data integrity.
Safety Data Sheet
You can download your Safety Data Sheet for LA020
For other languages please contact us: witega@witega.de
Additional information
| Chemical name | (2S,5R,6R)-6-[[(2R)-2-Amino-2-(4-hydroxyphenyl-13C6)acetyl]amino]-3,3-dimethyl-7-oxo-4-thia-1-azabicyclo[3.2.0]heptane-2-carboxylic acid |
|---|---|
| Molecular Formula | C1013C6H19N3O5S |
| Molecular Weight | 371.36 g/mol |
| Isotopic purity | 99.1 atom% 13C (MS) |
| HPLC purity | > 98.0 % |
| Overall purity | > 98.0 % (HPLC) |
| Product Format | Neat |
| Delivery time | In stock |
| shelf life | 24 months |
| Storage | -20°C |
| Country of Origin | Germany |
| Product No. | LA020 |
| CAS Reg. No. | not available |
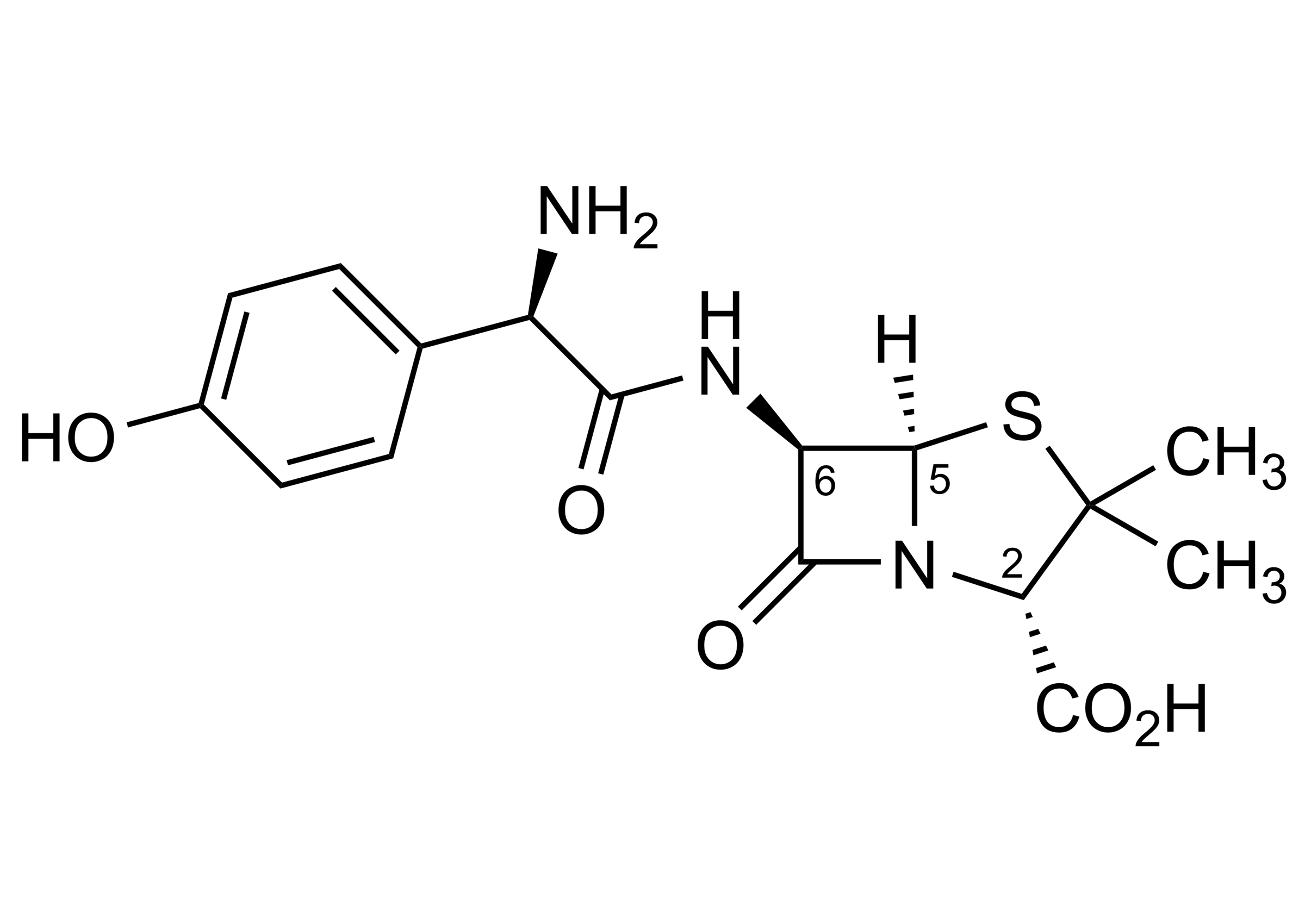
| Alternate CAS Reg. No. | 26787-78-0 (unlabelled compound) |
| Offer | 5 mg, 10 mg |