| Product No. | CH052 |
|---|---|
| CAS Reg. No. | 112398-08-0 |
| Alternate CAS Reg. No. | - |
| Offer | 100 mg |
112398-08-0
- Documentation
- Details
Chemical name
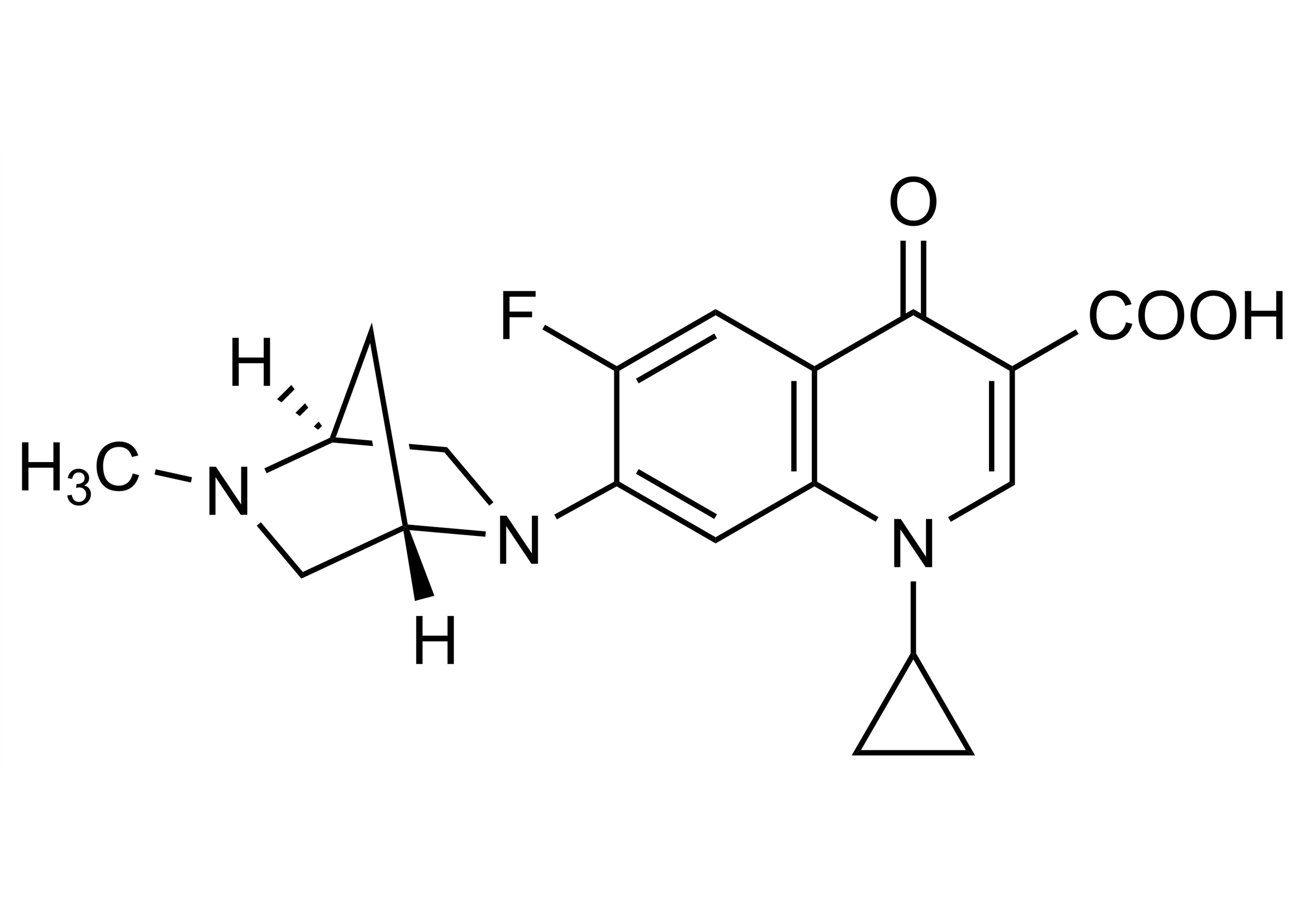
1-Cyclopropyl-6-fluoro-7-[(1S,4S)-5-methyl-2,5-diazabicyclo-[2.2.1]hept-2-yl]-4-oxo-1,4-dihydroquinoline-3-carboxylic acid
Description
Danofloxacin (CAS 112398-08-0) reference standard supports accurate LC-MS/MS and GC-MS quantification from the first injection. This fluoroquinolone reference compound is produced and distributed by WITEGA Laboratorien Berlin-Adlershof GmbH and enables traceable calibration, robust method validation, and confident confirmatory analysis. With consistent purity and defined content, laboratories can streamline workflows, reduce reanalysis, and document compliance with ease.
Engineered for routine and research settings alike, this reference standard helps you build solid calibration models and safeguard traceability throughout your analytical process. You can integrate it quickly into existing SOPs and meet stringent reporting needs.
- High purity and defined assay for reproducible quantitative results
- Batch-specific documentation and traceability for audits and quality systems
- Optimized for LC-MS/MS and GC-MS workflows in complex matrices
- Reliable performance for calibration curves, QC checks, and confirmation
Typical applications span regulated and exploratory environments where decision-ready data matters:
- Regulated laboratories performing residue monitoring and release testing
- Pharmaceutical research and development
- Veterinary and food residue control programs
- Metabolism and pharmacokinetic studies
- Multi-residue method development and verification
Use this reference standard to prepare calibration solutions, spike matrix-matched samples, and verify recoveries over the working range. Then, confirm identity through retention time and MRM transitions while tracking intermediate precision. Furthermore, routine QC samples help maintain traceability over time. Choose Danofloxacin (CAS 112398-08-0) as your reference standard to strengthen quantification, validation, and confirmatory analysis. For procurement and technical support, contact WITEGA Laboratorien Berlin-Adlershof GmbH.
Safety Data Sheet
You can download your Safety Data Sheet for CH052
For other languages please contact us: witega@witega.de
Additional information
| Chemical name | 1-Cyclopropyl-6-fluoro-7-[(1S,4S)-5-methyl-2,5-diazabicyclo-[2.2.1]hept-2-yl]-4-oxo-1,4-dihydroquinoline-3-carboxylic acid |
|---|---|
| Molecular Formula | C19H20FN3O3 |
| Molecular Weight | 357.38 g/mol |
| Isotopic purity | - |
| HPLC purity | > 99.0 % |
| Overall purity | > 99.0 % (HPLC) |
| Product Format | Neat |
| Delivery time | In stock |
| shelf life | 24 months |
| Storage | refrigerator, 2-8°C |
| Country of Origin | Germany |
| Product No. | CH052 |
| CAS Reg. No. | 112398-08-0 |
| Alternate CAS Reg. No. | – |
| Offer | 100 mg |