| Product No. | LA012 |
|---|---|
| CAS Reg. No. | 104557-24-6 |
| Alternate CAS Reg. No. | - |
| Offer | 10 mg |
104557-24-6
- Documentation
- Details
Chemical name
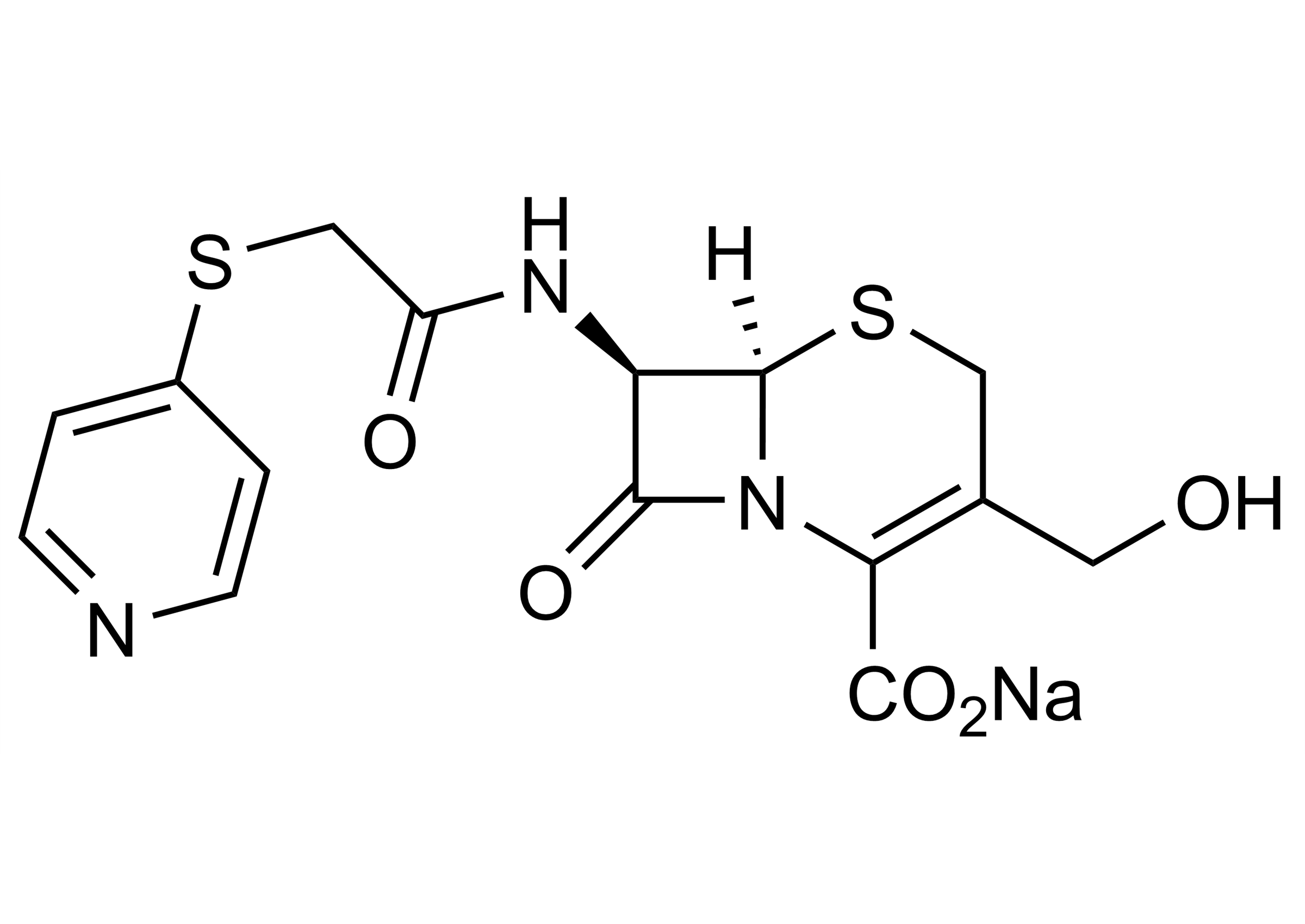
(6R,7R)-3-Hydroxymethyl-8-oxo-7-[(4-pyridinylthio)acetyl]amino-5-thia-1-aza-bicyclo[4.2.0]oct-2-ene-2-carboxylic acid sodium salt
Description
Desacetylcefapirin sodium salt (CAS 104557-24-6) is a high-purity reference standard produced and distributed by WITEGA Laboratorien Berlin-Adlershof GmbH. It supports LC-MS/MS quantification and GC-MS confirmatory analysis with robust traceability to certified reference material. Laboratories use it as a residue control standard during method validation for veterinary drugs and multi-residue method development. These qualities benefit regulated laboratories quality control and pharmaceutical research cephalosporin metabolite studies.
This reference standard delivers consistent performance across calibration, verification, and confirmatory workflows. Moreover, it assists analysts in establishing linear calibration, determining limits of detection, and verifying matrix effects. As a metabolite of a beta-lactam antibiotic, it helps confirm identity through retention time and ion ratio criteria in LC-MS/MS, and it enhances confidence in GC-MS screens when derivatization strategies are applied.
Key advantages:
- Produced and distributed by WITEGA Laboratorien Berlin-Adlershof GmbH with documented lot-specific CoA.
- Designed for calibration, traceability, and method validation in demanding workflows.
- Optimized for LC-MS/MS and compatible with GC-MS confirmatory analysis.
- Suitable for matrix-matched calibration and routine quality control checks.
Typical applications:
- Residue control in food, feed, and environmental matrices.
- Metabolism studies and kinetic investigations for cephalosporins.
- Pharmaceutical research on beta-lactam pathways and degradation products.
- Multi-residue method development and verification across instrument platforms.
- Confirmatory analysis in regulated laboratories following established guidelines.
Use this reference standard to prepare primary stock solutions for calibration curves, bracketing standards, and independent quality control samples. Additionally, apply it in spike-and-recovery experiments to assess trueness and precision. Therefore, your laboratory can maintain traceable quantification, verify selectivity, and ensure reliable confirmatory analysis throughout the full analytical lifecycle. For procurement or technical documentation, please contact WITEGA Laboratorien Berlin-Adlershof GmbH.
Safety Data Sheet
You can download your Safety Data Sheet for LA012
For other languages please contact us: witega@witega.de
Additional information
| Chemical name | (6R,7R)-3-Hydroxymethyl-8-oxo-7-[(4-pyridinylthio)acetyl]amino-5-thia-1-aza-bicyclo[4.2.0]oct-2-ene-2-carboxylic acid sodium salt |
|---|---|
| Molecular Formula | C15H14N3NaO5S2 |
| Molecular Weight | 403.41 g/mol |
| Isotopic purity | - |
| HPLC purity | > 97.0 % |
| Overall purity | > 92.0 ± 0.2 % |
| Product Format | Neat |
| Delivery time | In stock |
| shelf life | 24 months |
| Storage | -20°C |
| Country of Origin | Germany |
| Product No. | LA012 |
| CAS Reg. No. | 104557-24-6 |
| Alternate CAS Reg. No. | – |
| Offer | 10 mg |