| Product No. | LA025 |
|---|---|
| CAS Reg. No. | 13412-64-1 |
| Alternate CAS Reg. No. | - |
| Offer | 100 mg |
13412-64-1
- Documentation
- Details
Chemical name
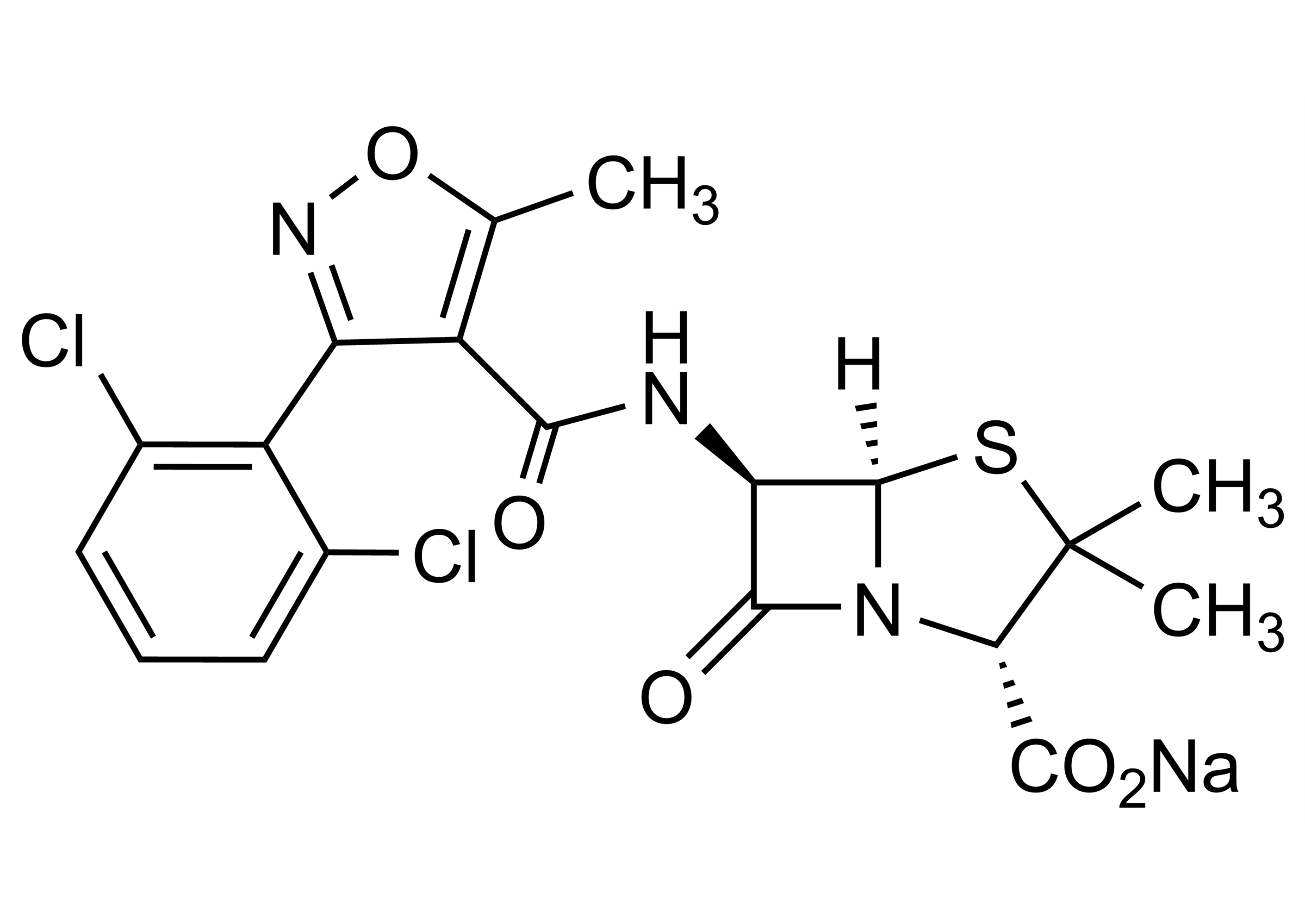
(2S,5R,6R)-6-[[[3-(2,6-Dichlorophenyl)-5-methyl-4-isoxazolyl]carbonyl]amino]-3,3-dimethyl-7-oxo-4-thia-1-azabicyclo[3.2.0]heptane-2-carboxylic acid sodium salt
Description
Dicloxacillin sodium salt (CAS 13412-64-1) is a high-purity reference standard produced and distributed by WITEGA Laboratorien Berlin-Adlershof GmbH. It supports LC-MS/MS and GC-MS analytical quantification in complex matrices. This beta lactam antibiotic reference standard enables precise calibration and reliable confirmatory analysis across demanding workflows. Moreover, lot-specific documentation ensures transparency and strong data integrity from method development to routine testing.
Designed for traceability, the material facilitates calibration and system suitability checks. It also streamlines method validation with well-characterized composition and consistent performance. Therefore, analysts can establish defensible reporting limits and confident identification in both targeted and non-targeted approaches. The reference standard helps reduce variability, improve linearity, and maintain long-term comparability between laboratories.
Typical use cases include regulated laboratories, pharmaceutical research programs, residue control studies, and metabolism investigations. Additionally, the material fits multi-residue method development where robustness and selectivity matter. Dicloxacillin sodium salt (CAS 13412-64-1) integrates seamlessly into LC-MS/MS and GC-MS workflows, from sample preparation optimization to confirmatory analysis.
Every batch from WITEGA Laboratorien Berlin-Adlershof GmbH is prepared with rigorous quality procedures and comes with comprehensive documentation. You can use it to verify instrument response, monitor recovery, and confirm retention and mass transitions. Furthermore, it aids uncertainty assessment and supports audits through clear metrological traceability.
- Supports calibration curves for quantitative LC-MS/MS and GC-MS workflows
- Enables method validation and confirmatory analysis with robust traceability
- Suitable for pharmaceutical research, residue control, and metabolism studies
- Valuable in multi-residue method development and inter-laboratory comparability
- Produced and distributed by WITEGA Laboratorien Berlin-Adlershof GmbH with lot-specific documentation
Choose this reference standard to build reliable calibration models, verify selectivity, and maintain consistent results over time. For availability, documentation, or technical support, please contact WITEGA Laboratorien Berlin-Adlershof GmbH.
Safety Data Sheet
You can download your Safety Data Sheet for LA025
For other languages please contact us: witega@witega.de
Additional information
| Chemical name | (2S,5R,6R)-6-[[[3-(2,6-Dichlorophenyl)-5-methyl-4-isoxazolyl]carbonyl]amino]-3,3-dimethyl-7-oxo-4-thia-1-azabicyclo[3.2.0]heptane-2-carboxylic acid sodium salt |
|---|---|
| Molecular Formula | C19H16Cl2N3NaO5S |
| Molecular Weight | 492.31 g/mol |
| Isotopic purity | - |
| HPLC purity | > 99.0 % |
| Overall purity | > 87.0 % (NMR internal standard) |
| Product Format | Neat |
| Delivery time | In stock |
| shelf life | 24 months |
| Storage | -20°C |
| Country of Origin | Germany |
| Product No. | LA025 |
| CAS Reg. No. | 13412-64-1 |
| Alternate CAS Reg. No. | – |
| Offer | 100 mg |