| Product No. | ST034 |
|---|---|
| CAS Reg. No. | 88247-87-4; 792214-66-5 |
| Alternate CAS Reg. No. | 10418-03-8 (unlabelled compound) |
| Offer | 2.5 mg, 10 mg |
88247-87-4; 792214-66-5
- Documentation
- Details
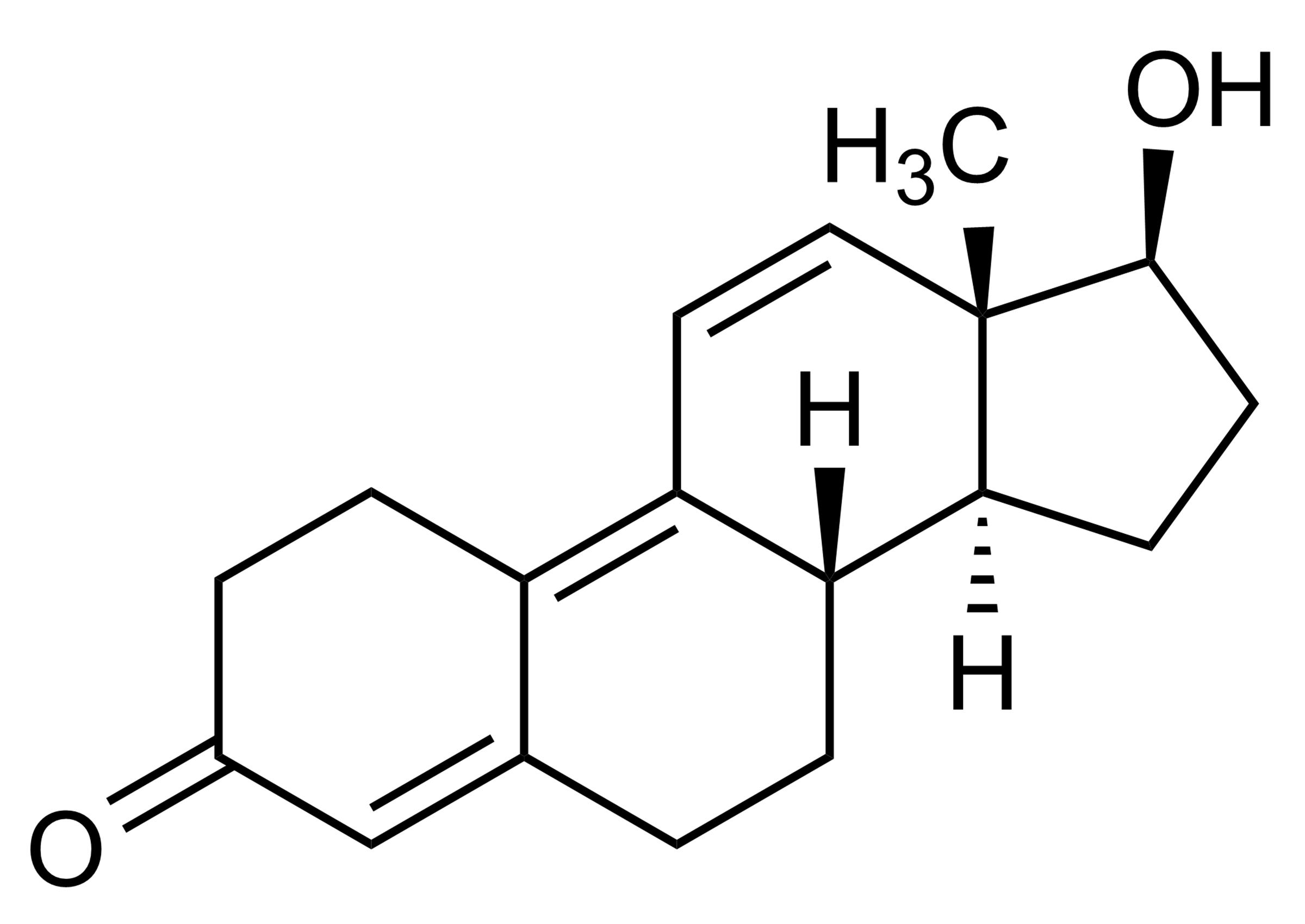
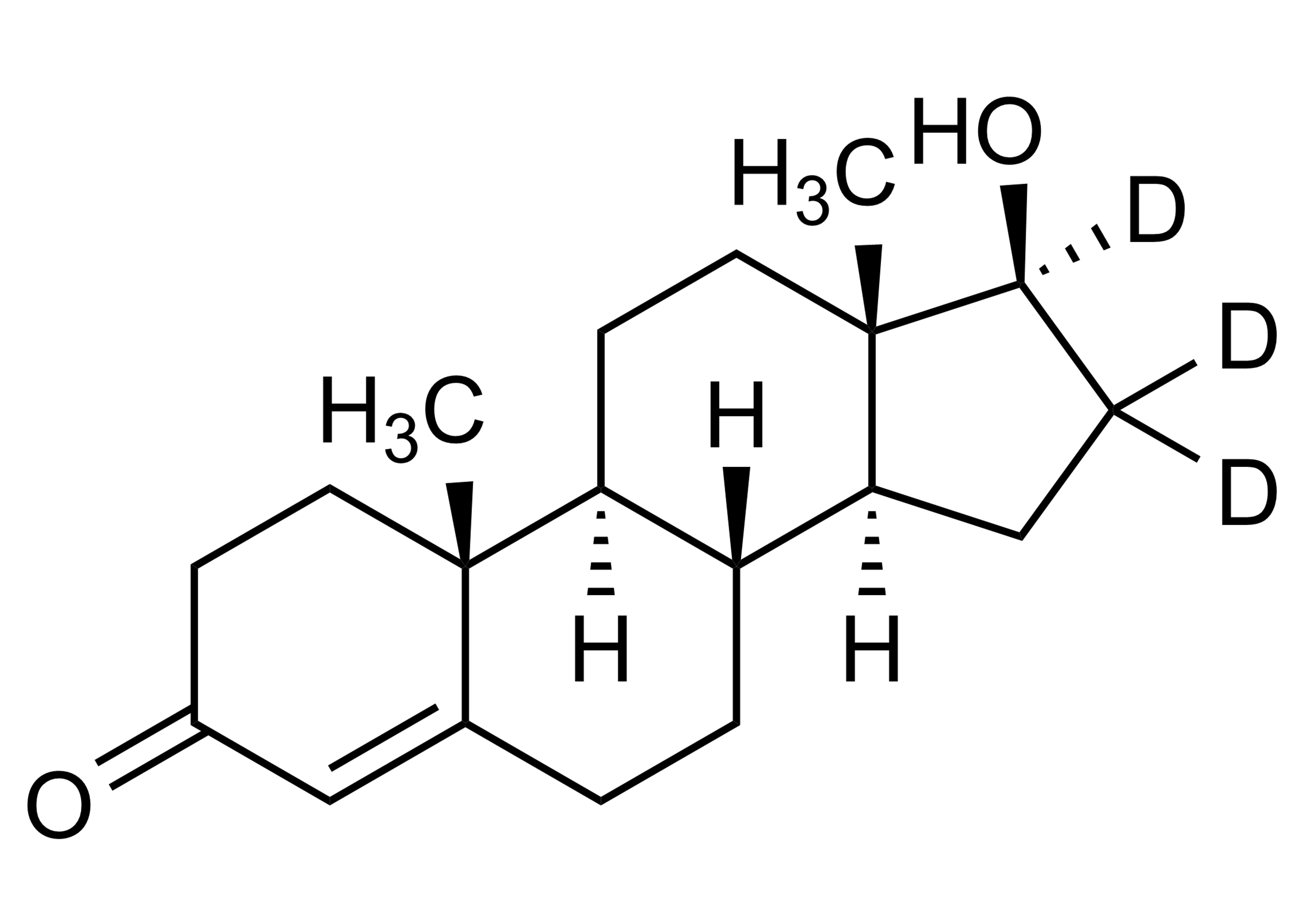
Chemical name
(17β)-17-(Methyl-D3)-2’H-androst-2-eno[3,2-c]pyrazol-17-ol; n(5α,17β)-17-(Methyl-D3)-1′H-androstano[3,2-c]pyrazol-17-ol
Description
Stanozolol-D3 (CAS 88247-87-4) is an anabolic steroid reference standard produced and distributed by WITEGA Laboratorien Berlin-Adlershof GmbH for precise LC-MS/MS and GC-MS workflows. It supports traceable calibration, robust method validation, and confident confirmatory analysis. Therefore, laboratories can quantify residues reliably and comply with regulatory requirements. For documentation needs, this reference standard includes complete product data and recommended handling guidance.
Designed for performance, this Stanozolol-D3 reference standard helps achieve low limits of quantification and consistent recovery across complex matrices. It provides lot-to-lot reproducibility, making routine checks straightforward. Additionally, it assists in matrix effect evaluation and instrument suitability testing.
- Optimized for LC-MS/MS and GC-MS quantification
- Traceability ensures credible, audit-ready calibration
- Reliable support for method validation and verification
- Fit for confirmatory analysis in regulatory contexts
- High chemical purity and well-documented identity
Typical use cases include regulated laboratories, pharmaceutical research, residue control programs, metabolism studies, and multi-residue method development. Scientists benefit from stable signal response, clear isotope differentiation, and consistent measurement across batches. Furthermore, the material aids in retention time tracking and system precision assessments.
- Residue control in food and sports testing
- Metabolism and excretion pathway studies
- Development of multi-residue methods
- Calibration and quality control for quantitative analyses
- Confirmatory analysis with stringent identification criteria
This reference standard from WITEGA Laboratorien Berlin-Adlershof GmbH comes with comprehensive documentation for traceability. In addition, information on solubility and recommended solvents simplifies solution preparation. Ultimately, Stanozolol-D3 strengthens data integrity and ensures confident, reproducible results across LC-MS/MS or GC-MS platforms.
Safety Data Sheet
You can download your Safety Data Sheet for ST034
For other languages please contact us: witega@witega.de
Additional information
| Chemical name | (17β)-17-(Methyl-D3)-2’H-androst-2-eno[3,2-c]pyrazol-17-ol; n(5α,17β)-17-(Methyl-D3)-1′H-androstano[3,2-c]pyrazol-17-ol |
|---|---|
| Molecular Formula | C21H29D3N2O |
| Molecular Weight | 331.51 g/mol |
| Isotopic purity | 99.8 atom% D (MS) |
| HPLC purity | > 99.0 % |
| Overall purity | > 99.0 % (HPLC) |
| Product Format | Neat |
| Delivery time | In stock |
| shelf life | 24 months |
| Storage | refrigerator, 2-8°C |
| Country of Origin | Germany |
| Product No. | ST034 |
| CAS Reg. No. | 88247-87-4; 792214-66-5 |
| Alternate CAS Reg. No. | 10418-03-8 (unlabelled compound) |
| Offer | 2.5 mg, 10 mg |