| Product No. | SA005 |
|---|---|
| CAS Reg. No. | 1196157-72-8 |
| Alternate CAS Reg. No. | 72-14-0 (unlabelled compound) |
| Offer | 25 mg, 50 mg |
1196157-72-8
- Documentation
- Details
Chemical name
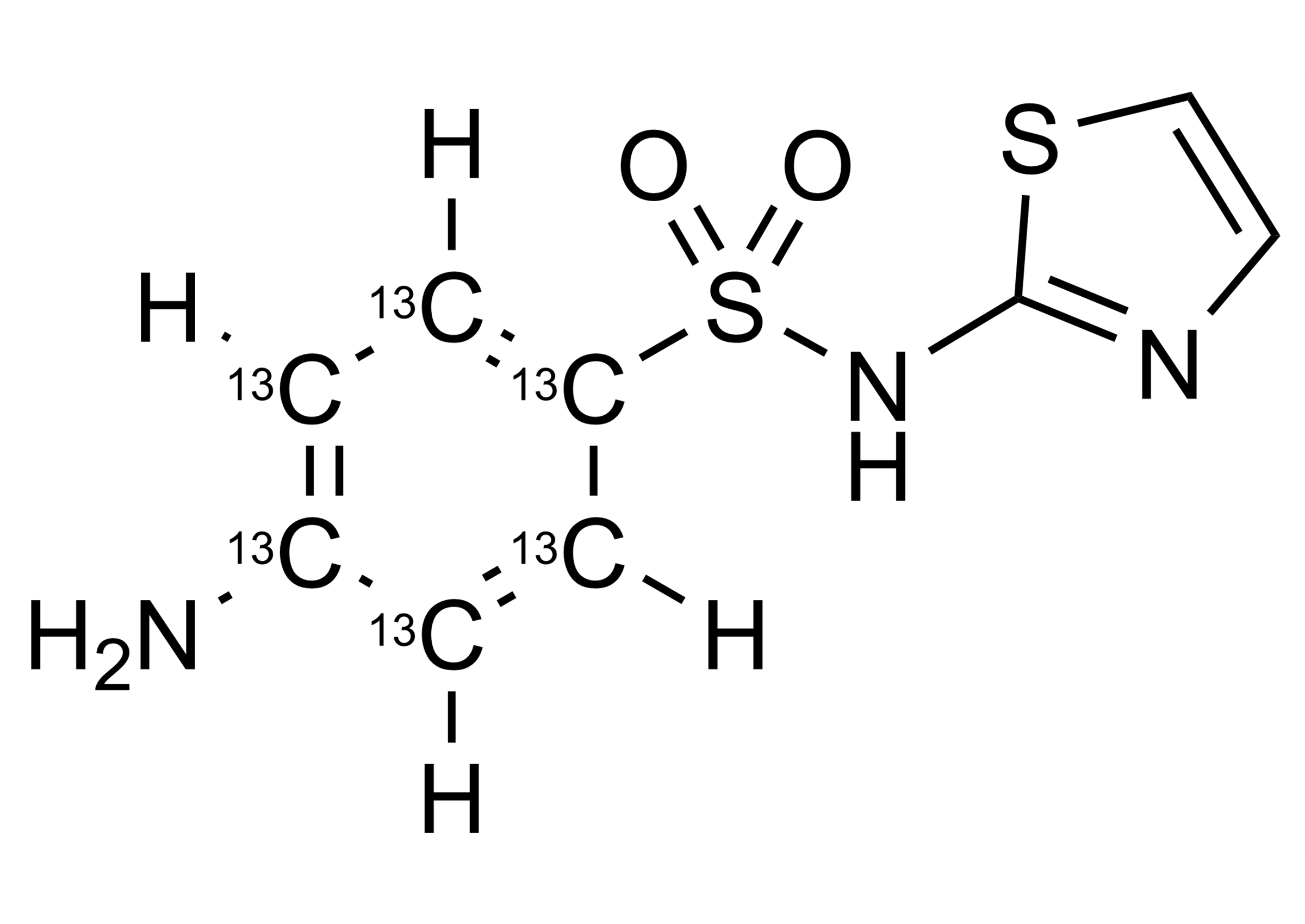
4-Amino-N-thiazol-2-yl-benzene-13C6-sulfonamide
Description
Sulfathiazole-13C6 (CAS 1196157-72-8) is a high-purity reference standard produced and distributed by WITEGA Laboratorien Berlin-Adlershof GmbH. It enables robust LC-MS/MS and GC-MS quantification, ensures traceability, and simplifies calibration. It also supports method validation and precise confirmatory analysis in routine and research settings. Keywords: stable isotope labeled antibiotic standard, LC-MS/MS calibration solution, antibacterial residue analysis, mass spectrometry reference material, traceable calibration compound, method validation standard, confirmatory analysis reference, veterinary drug residue testing, multi-residue method development, regulated laboratory quality control.
This isotopically labeled reference standard improves selectivity in complex matrices, which strengthens identification and quantification. Use it to build linear, matrix-matched calibration curves and to evaluate recovery. Moreover, it supports cross-lab comparability and system suitability testing. You can check instrument response, monitor drift, and confirm identification criteria with confidence.
- Traceable documentation and lot-specific certificate of analysis.
- Optimized for LC-MS/MS and GC-MS workflows.
- Suitable for calibration, quality control, and system suitability checks.
- Consistent performance in multi-residue methods across matrices.
- Stable composition for reliable use when stored as instructed.
Typical applications include regulated laboratories, pharmaceutical research programs, residue control campaigns, metabolism studies, and multi-residue method development for antimicrobial surveillance. The design supports precise quantification and transparent data defensibility, even under stringent compliance requirements.
- Regulated laboratories and compliance studies.
- Pharmaceutical research and development.
- Residue control in food, feed, and environmental samples.
- Metabolism and transformation investigations.
- Multi-residue method development and validation.
The Sulfathiazole-13C6 (CAS 1196157-72-8) reference standard from WITEGA Laboratorien Berlin-Adlershof GmbH ships with a certificate of analysis detailing identity, purity, and assigned value with uncertainty. Clear storage and handling recommendations help maintain integrity and traceable performance. Choose this reference standard to support calibration, method validation, and confirmatory analysis with dependable results.
Safety Data Sheet
You can download your Safety Data Sheet for SA005
For other languages please contact us: witega@witega.de
Additional information
| Chemical name | 4-Amino-N-thiazol-2-yl-benzene-13C6-sulfonamide |
|---|---|
| Molecular Formula | C313C6H9N3O2S2 |
| Molecular Weight | 261.25 g/mol |
| Isotopic purity | >99.0 atom% 13C (1H NMR) |
| HPLC purity | > 99.0 % |
| Overall purity | > 99.0 % (HPLC) |
| Product Format | Neat |
| Delivery time | In stock |
| shelf life | 24 months |
| Storage | refrigerator, 2-8°C |
| Country of Origin | Germany |
| Product No. | SA005 |
| CAS Reg. No. | 1196157-72-8 |
| Alternate CAS Reg. No. | 72-14-0 (unlabelled compound) |
| Offer | 25 mg, 50 mg |