| Product No. | TC001 |
|---|---|
| CAS Reg. No. | 220620-09-7 |
| Alternate CAS Reg. No. | - |
| Offer | 10 mg |
220620-09-7
- Documentation
- Details
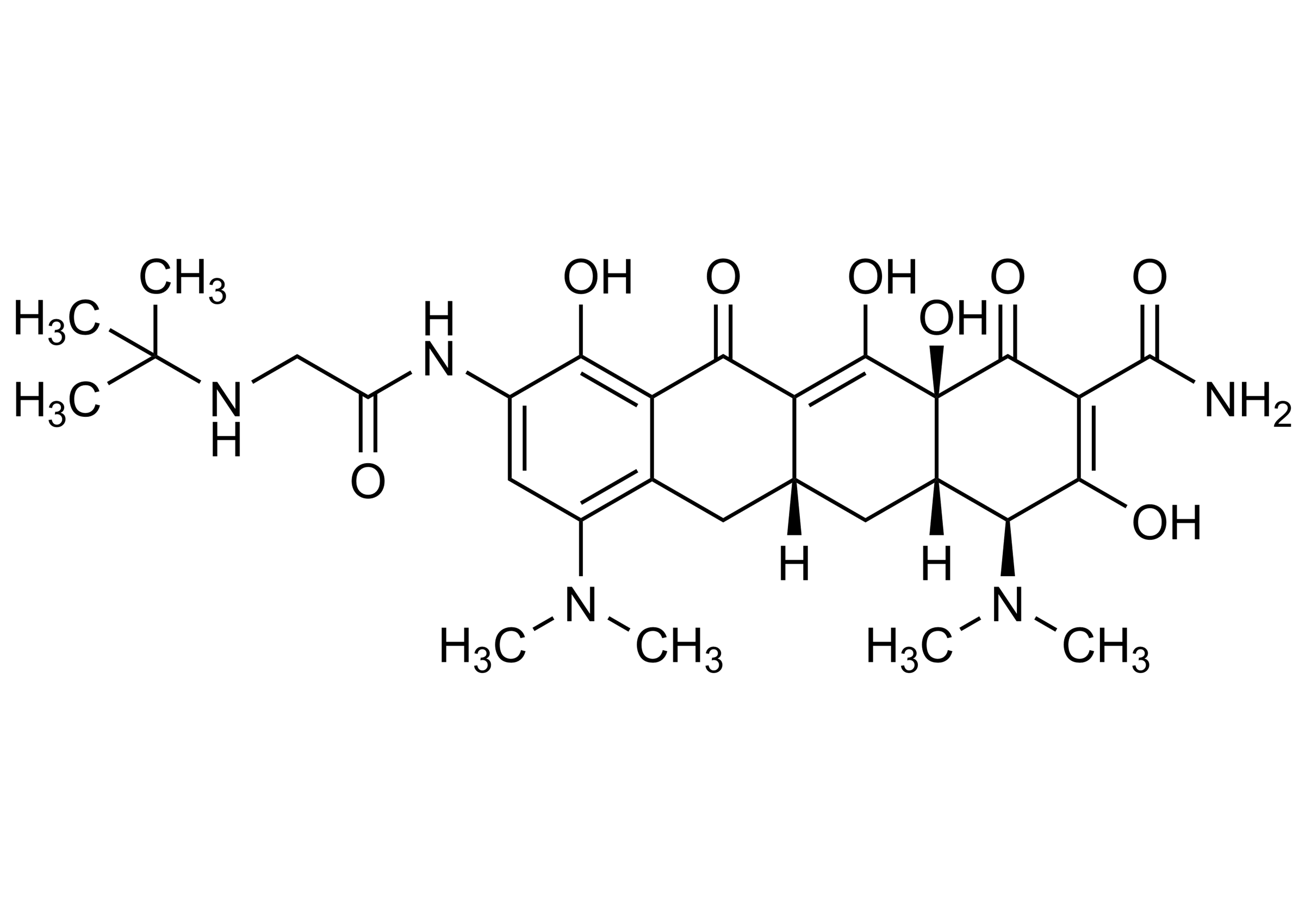
Chemical name
(4S,4aS,5aR,12aS)-4,7-Bis(dimethylamino)-9-[[2-[(1,1-dimethylethyl)amino] acetyl]amino]-1,11-dioxo-1,4,4a,5,5a,6,11,12a-octahydro-3,10,12,12a-tetrahydroxy-2-naphthacenecarboxamide
Description
Tigecycline (CAS 220620-09-7) reference standard empowers robust LC-MS/MS and GC-MS quantification from the first injection to final report. Produced and distributed by WITEGA Laboratorien Berlin-Adlershof GmbH, it supports analytical traceability, calibration, method validation, and confirmatory analysis. As an LC-MS/MS reference material and a traceable calibration solution, it helps regulated laboratories, pharmaceutical research teams, residue control programs, metabolism studies, and multi-residue method development achieve consistent, defensible results.
Developed for reliable performance, this reference standard provides stable, consistent response across instruments and matrices. Moreover, its batch-specific documentation enables seamless integration into SOPs and audits. You gain confidence in calibration curves, spiking experiments, and long-term trending.
- High chemical purity and well-defined identity for accurate quantification
- Traceable batch documentation and ready-to-use data for compliance
- Suitable for external calibration, recovery checks, and system suitability
- Proven compatibility with LC-MS/MS and GC-MS workflows
- Supports confirmatory analysis with precise retention and ion ratio targets
- Clear handling guidance for storage, dilution, and stability planning
Typical applications include:
- Regulated laboratories performing routine screening and targeted confirmation
- Pharmaceutical research and development, including metabolism and degradation studies
- Residue control and surveillance programs with demanding reporting limits
- Multi-residue method development requiring reproducible calibration hierarchies
- Cross-lab harmonization where traceability and comparability matter
In practice, prepare a stock solution, build multi-level external calibration, and verify recovery in representative matrices. Then, use matrix-matched calibrants to evaluate ion suppression. Finally, confirm targets with qualifier transitions and retention criteria. Throughout these steps, Tigecycline (CAS 220620-09-7) reference standard helps maintain measurement integrity. For supply, documentation, and technical support, contact WITEGA Laboratorien Berlin-Adlershof GmbH.
Safety Data Sheet
You can download your Safety Data Sheet for TC001
For other languages please contact us: witega@witega.de
Additional information
| Chemical name | (4S,4aS,5aR,12aS)-4,7-Bis(dimethylamino)-9-[[2-[(1,1-dimethylethyl)amino] acetyl]amino]-1,11-dioxo-1,4,4a,5,5a,6,11,12a-octahydro-3,10,12,12a-tetrahydroxy-2-naphthacenecarboxamide |
|---|---|
| Molecular Formula | C29H39N5O8 |
| Molecular Weight | 585.65 g/mol |
| Isotopic purity | - |
| HPLC purity | > 99.0 % |
| Overall purity | > 99.0 % (HPLC) |
| Product Format | Neat |
| Delivery time | In stock |
| shelf life | 24 months |
| Storage | refrigerator, 2-8°C |
| Country of Origin | Germany |
| Product No. | TC001 |
| CAS Reg. No. | 220620-09-7 |
| Alternate CAS Reg. No. | – |
| Offer | 10 mg |