| Product No. | CH029 |
|---|---|
| CAS Reg. No. | 115964-29-9 |
| Alternate CAS Reg. No. | - |
| Offer | 10 mg, 25 mg |
115964-29-9
- Documentation
- Details
Chemical name
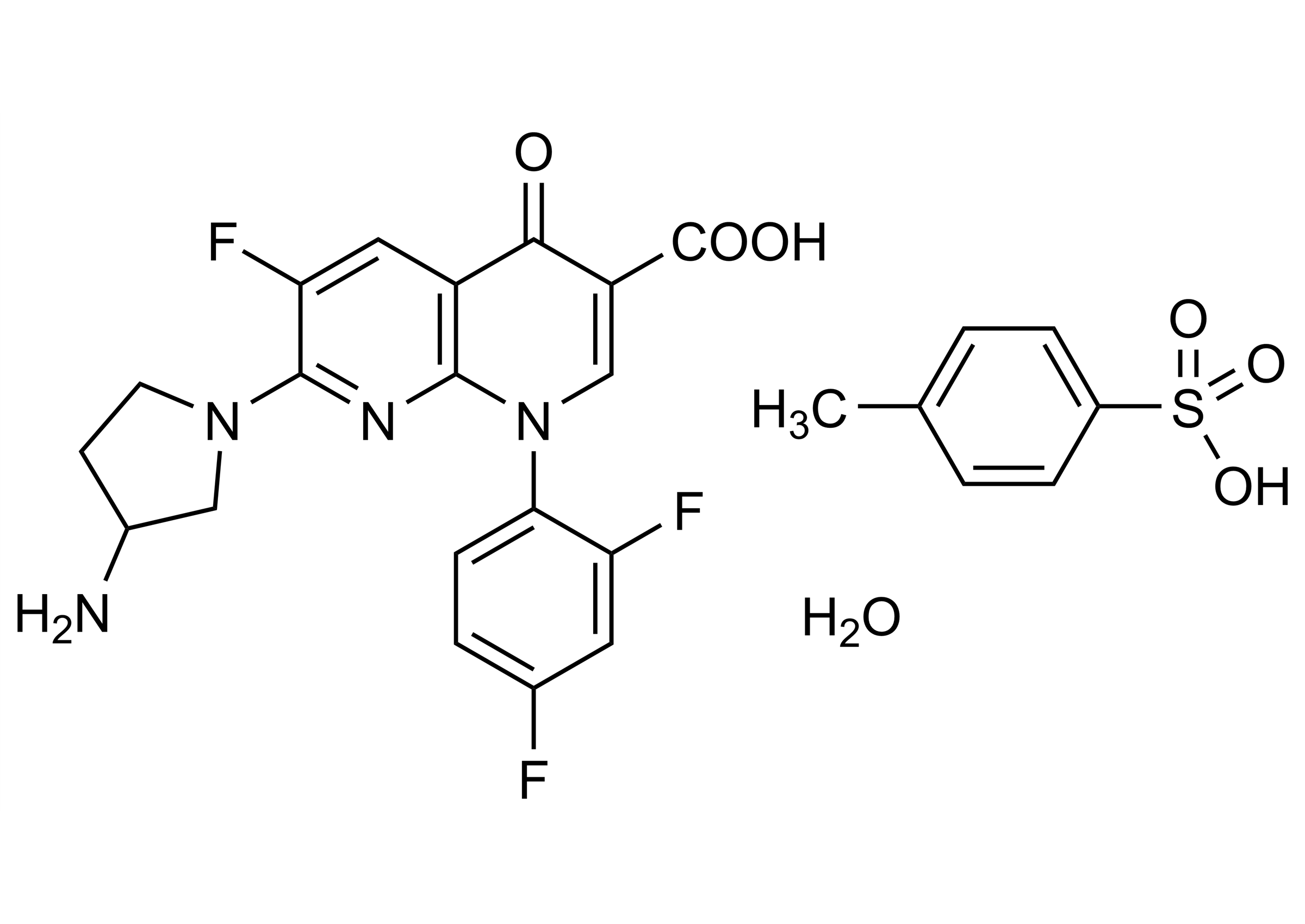
7-(3-Aminopyrrolidin-1-yl)-1-(2,4-difluorophenyl)-6-fluoro-1,4-dihydro-4-oxo-1,8-naphthyridine-3-carboxylic acid tosylate hydrate
Description
Tosufloxacin toluenesulfonate hydrate (CAS 115964-29-9) is a high-quality reference standard produced and distributed by WITEGA Laboratorien Berlin-Adlershof GmbH. It supports demanding LC-MS/MS calibration solution workflows as well as GC-MS confirmation material tasks. As a fluoroquinolone reference standard and a traceable analytical standard, it enables accurate quantification and robust quality control from screening to confirmation. Moreover, WITEGA provides lot-specific documentation to streamline compliance and reporting.
This reference standard is optimized for LC-MS/MS and GC-MS analytical quantification. It delivers reliable calibration, method validation, and confirmatory analysis across a wide range of matrices. Therefore, laboratories can establish traceability to support defensible results and harmonized reporting. Tosufloxacin toluenesulfonate hydrate (CAS 115964-29-9) comes with purity information and handling guidance to help maintain integrity over the entire analytical workflow.
- Purpose-built reference standard with consistent quality for mass spectrometry.
- Supports calibration, system suitability, and instrument performance checks.
- Enables quantitative method validation and confirmatory analysis.
- Backed by traceable documentation and batch transparency.
- Compatible with typical solvent systems for LC-MS/MS and GC-MS.
- Clear labeling featuring Tosufloxacin toluenesulfonate hydrate (CAS 115964-29-9) for easy inventory control.
Typical use cases include regulated laboratories that need traceable calibration, pharmaceutical research focused on fluoroquinolone characterization, residue control programs, and metabolism studies. Additionally, multi-residue method development benefits from consistent recovery, linearity, and ruggedness testing with this reference standard.
- Regulatory monitoring and proficiency testing.
- Pharmaceutical discovery and development support.
- Residue control and surveillance methods.
- In vitro and in vivo metabolism studies.
- Matrix-matched calibration in complex samples.
- Method transfer and cross-lab harmonization.
Use this material to build robust calibration curves, verify accuracy, and assess precision. Store according to the accompanying guidance to preserve stability. With this reference standard from WITEGA Laboratorien Berlin-Adlershof GmbH, you can establish confidence in LC-MS/MS and GC-MS results from screening through final confirmation.
Safety Data Sheet
You can download your Safety Data Sheet for CH029
For other languages please contact us: witega@witega.de
Additional information
| Chemical name | 7-(3-Aminopyrrolidin-1-yl)-1-(2,4-difluorophenyl)-6-fluoro-1,4-dihydro-4-oxo-1,8-naphthyridine-3-carboxylic acid tosylate hydrate |
|---|---|
| Molecular Formula | C26H23F3N4O6S x H2O |
| Molecular Weight | 594.56 g/mol |
| Isotopic purity | - |
| HPLC purity | > 99.0 % |
| Overall purity | > 99.0 % (HPLC) |
| Product Format | Neat |
| Delivery time | In stock |
| shelf life | 24 months |
| Storage | refrigerator, 2-8°C |
| Country of Origin | Germany |
| Product No. | CH029 |
| CAS Reg. No. | 115964-29-9 |
| Alternate CAS Reg. No. | – |
| Offer | 10 mg, 25 mg |