| Product No. | OP291 |
|---|---|
| CAS Reg. No. | 21411-53-0 |
| Alternate CAS Reg. No. | - |
| Offer | 10 mg |
21411-53-0
- Documentation
- Details
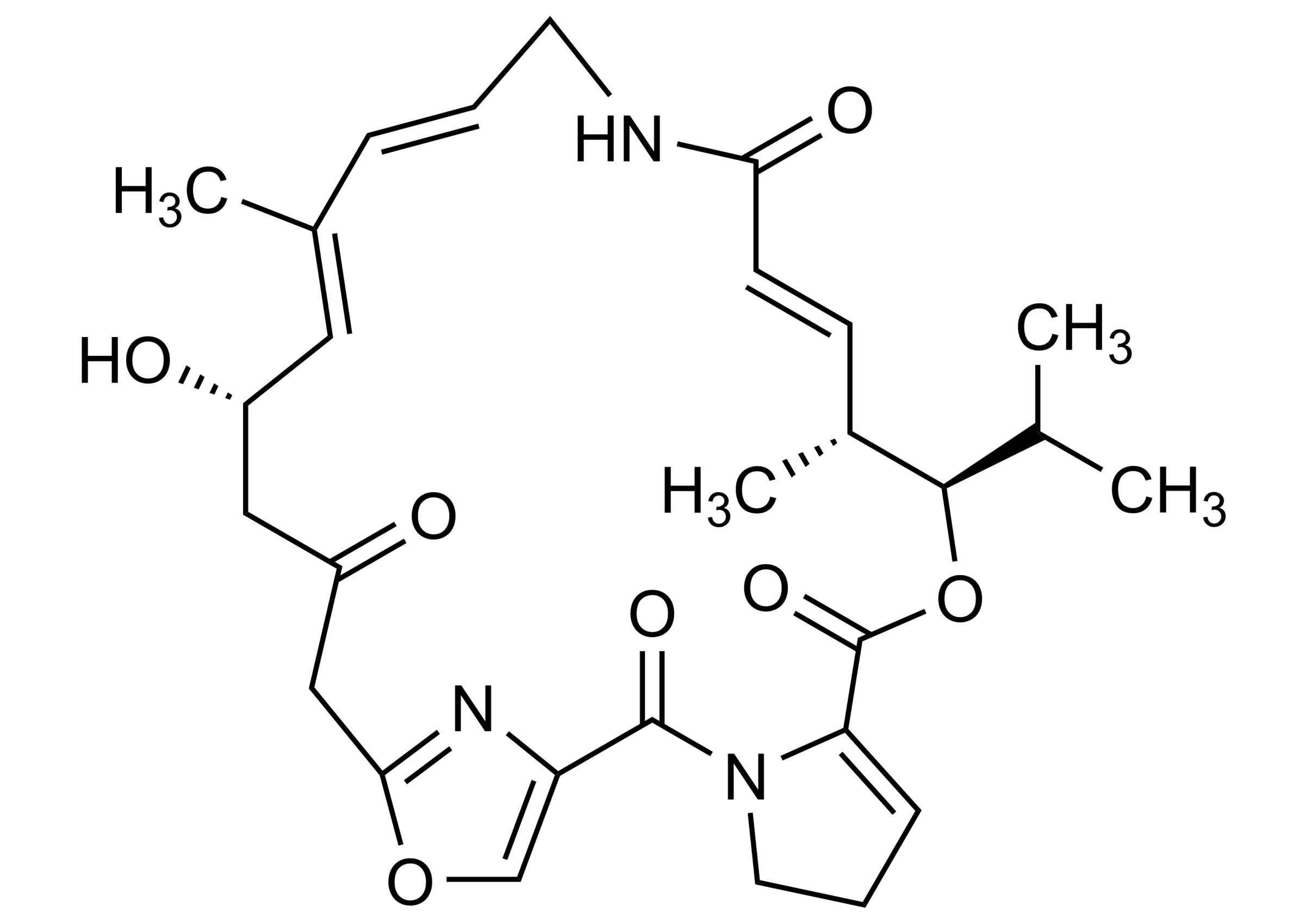
Chemical name
(3R,4R,5E,10E,12E,14S)-8,9,14,15,24,25-Hexahydro-14-hydroxy-4,12-dimethyl-3-(1-methylethyl)-3H-21,18-nitrilo-1H,22H-pyrrolo[2,1-c][1,8,4,19]dioxadiaza-cyclotetracosine-1,7,16,22(4H,17H)-tetrone
Description
Virginamycin M1 (CAS 21411-53-0) is a high-quality reference standard produced and distributed by WITEGA Laboratorien Berlin-Adlershof GmbH for LC-MS/MS and GC-MS workflows. Designed for accuracy and reliability, this material supports quantification, traceability, calibration, method validation, and confirmatory analysis. Therefore, it fits seamlessly into regulated methods as an antibiotic residue analysis standard, helping laboratories meet stringent performance criteria with confidence.
To ensure robust data, the reference standard is manufactured with strict quality controls and delivered with batch-specific documentation. It provides consistent response factors across instruments, making it suitable for calibration curves, matrix-matched quantitation, and quality control checks. Moreover, Virginamycin M1 (CAS 21411-53-0) enables reproducible recoveries and precise reporting limits.
- Supports LC-MS/MS and GC-MS quantification and calibration
- Optimized for method validation and confirmatory analysis
- Traceable production and detailed certification for audit readiness
- Excellent choice for multi-residue method development
- Reliable performance for complex matrices
Typical use cases include:
- Regulated laboratories performing official residue control
- Pharmaceutical research and discovery programs
- Food and feed monitoring, surveillance, and screening
- Metabolism studies and pathway elucidation
- Multi-residue method development and optimization
In practice, analysts use this reference standard to build linear calibration models, verify instrument response, and establish traceability for routine series. It assists in spike-and-recovery experiments, stability assessments, and system suitability testing. Furthermore, the material is suitable for setting decision limits and verifying measurement uncertainty during confirmatory analysis. Throughout your workflow, Virginamycin M1 (CAS 21411-53-0) helps maintain accuracy and compliance.
This reference standard is produced and distributed by WITEGA Laboratorien Berlin-Adlershof GmbH, ensuring dependable supply, expert support, and comprehensive documentation to meet your quality requirements.
Safety Data Sheet
You can download your Safety Data Sheet for OP291
For other languages please contact us: witega@witega.de
Additional information
| Chemical name | (3R,4R,5E,10E,12E,14S)-8,9,14,15,24,25-Hexahydro-14-hydroxy-4,12-dimethyl-3-(1-methylethyl)-3H-21,18-nitrilo-1H,22H-pyrrolo[2,1-c][1,8,4,19]dioxadiaza-cyclotetracosine-1,7,16,22(4H,17H)-tetrone |
|---|---|
| Molecular Formula | C28H35N3O7 |
| Molecular Weight | 525.59 g/mol |
| Isotopic purity | - |
| HPLC purity | > 99.0 % |
| Overall purity | > 99.0 % (HPLC) |
| Product Format | Neat |
| Delivery time | In stock |
| shelf life | 24 months |
| Storage | refrigerator, 2-8°C |
| Country of Origin | Germany |
| Product No. | OP291 |
| CAS Reg. No. | 21411-53-0 |
| Alternate CAS Reg. No. | – |
| Offer | 10 mg |