| Product No. | VS004 |
|---|---|
| CAS Reg. No. | 139110-80-8 |
| Alternate CAS Reg. No. | - |
| Offer | 10 mg |
139110-80-8
- Documentation
- Details
Chemical name
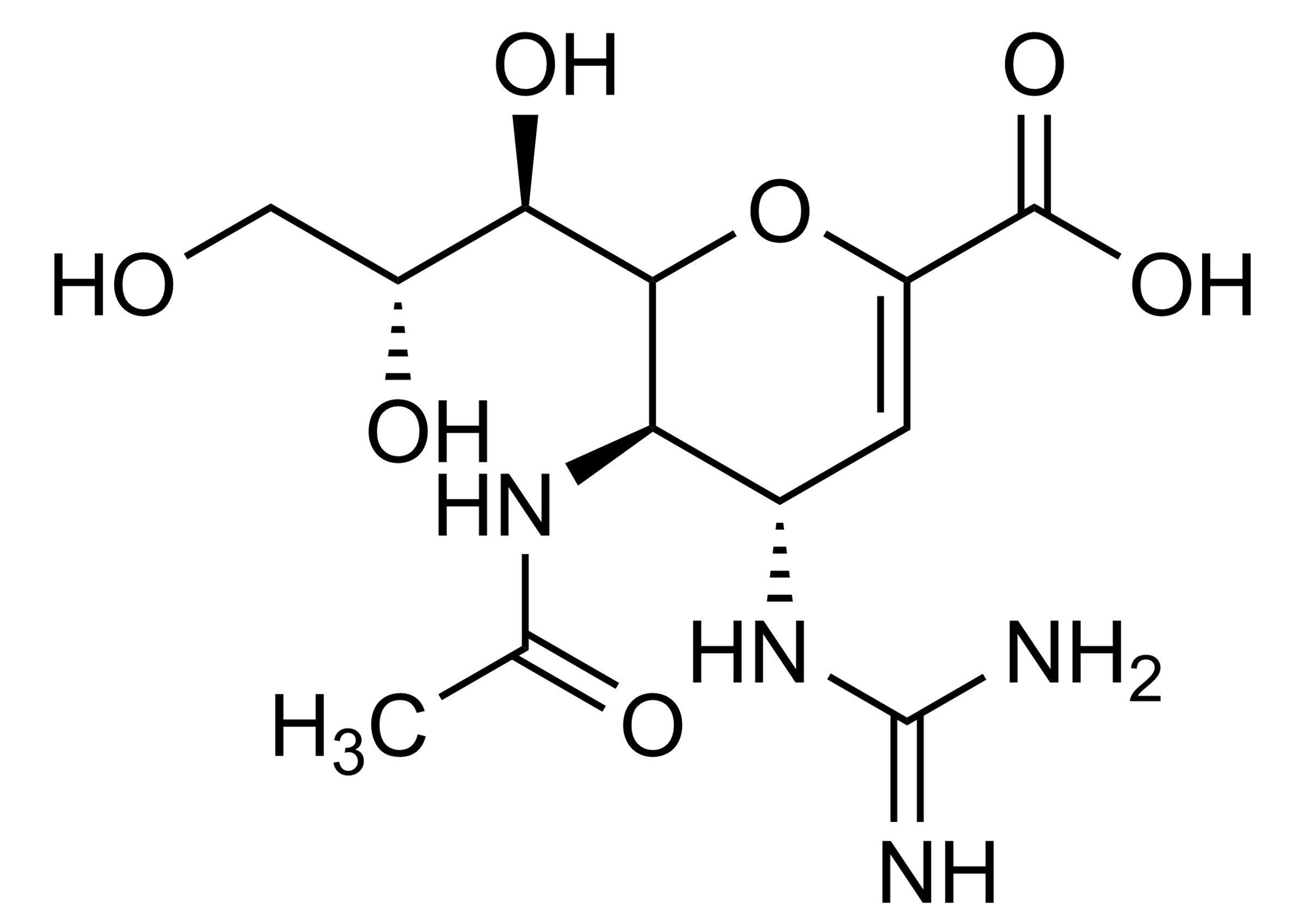
4-Guanidino-2,4-dideoxy-2,3-dehydro-N-acetylneuraminic acid
Description
Zanamivir (CAS 139110-80-8) reference standard supports robust LC-MS/MS and GC-MS workflows in regulated environments. Produced and distributed by WITEGA Laboratorien Berlin-Adlershof GmbH, it is designed for LC-MS/MS calibration solution preparation and confirmatory analysis reference material tasks. Typical uses span regulated laboratory compliance, neuraminidase inhibitor reference material applications, residue analysis quality control, metabolism study standard work, multi-residue method development, and pharmaceutical research traceability. Consequently, laboratories gain dependable performance and clear documentation for quantitative results.
This reference standard enables accurate analytical quantification, traceability, and calibration in daily routine and advanced studies. Moreover, it supports method validation from selectivity to linearity, as well as confirmatory analysis that meets stringent criteria. Each batch is manufactured to ensure consistency from lot to lot. In addition, the product can be applied across common solvent systems used in LC-MS/MS or GC-MS. Therefore, teams can streamline workflows, reduce re-analysis, and strengthen measurement confidence.
- Key applications: quantitative calibration and verification in LC-MS/MS and GC-MS
- Method validation: precision, accuracy, linearity, and robustness assessments
- Confirmatory analysis: unambiguous identification and result confirmation
- Regulated laboratories: compliance-driven residue and purity studies
- Pharmaceutical research: metabolism and pharmacokinetic investigations
- Multi-residue method development: screening and targeted quantification
- High purity and clear traceability for reliable calibration
- Lot-specific documentation and consistent quality control
- Optimized for routine and advanced confirmatory workflows
- Compatible with typical mobile phases and sample preparation steps
Choose this Zanamivir (CAS 139110-80-8) reference standard to improve reproducibility across projects, from initial feasibility to final reporting. Additionally, the material helps align data with quality systems and audit requirements. For specifications, documentation, and availability, please contact WITEGA Laboratorien Berlin-Adlershof GmbH.
Safety Data Sheet
You can download your Safety Data Sheet for VS004
For other languages please contact us: witega@witega.de
Additional information
| Chemical name | 4-Guanidino-2,4-dideoxy-2,3-dehydro-N-acetylneuraminic acid |
|---|---|
| Molecular Formula | C12H20N4O7 |
| Molecular Weight | 332.31 g/mol |
| Isotopic purity | - |
| HPLC purity | 98.9 ± 0.1 % |
| Overall purity | 98.9 ± 0.1 % (HPLC) |
| Product Format | Neat |
| Delivery time | In stock |
| shelf life | 24 months |
| Storage | refrigerator, 2-8°C |
| Country of Origin | Germany |
| Product No. | VS004 |
| CAS Reg. No. | 139110-80-8 |
| Alternate CAS Reg. No. | – |
| Offer | 10 mg |