| Product No. | ST038 |
|---|---|
| CAS Reg. No. | not available |
| Alternate CAS Reg. No. | 439791-84-1; 125590-76-3 (unlabelled compound) |
| Offer | 2.5 mg, 5 mg, 10 mg |
not available
- Documentation
- Details
Chemical name
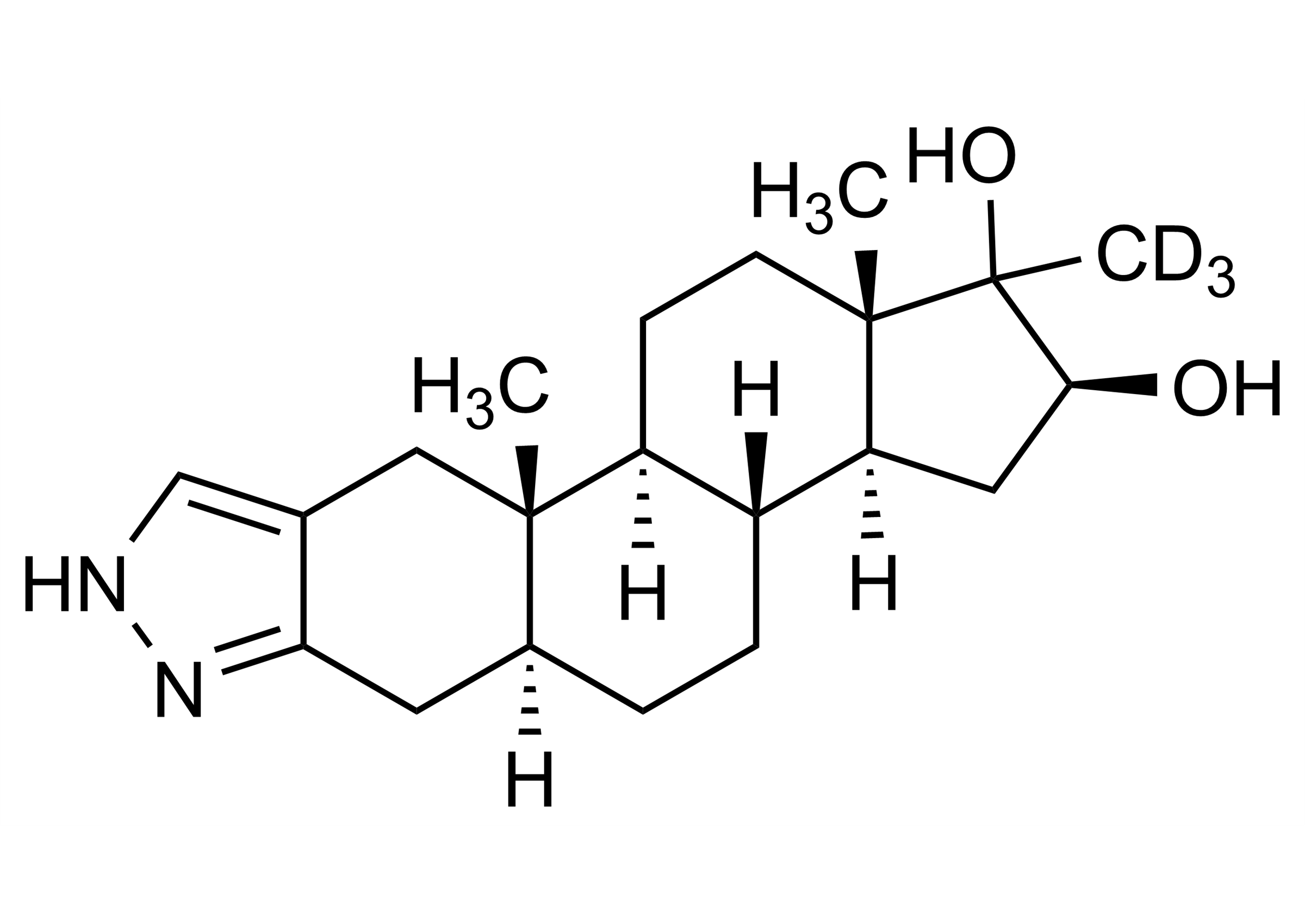
(5α,16β)-17-Methyl-D3-1’H-androstano[3,2-c]pyrazol-16,17-diol(5α,16β)-17-Methyl-D3-2’H-androst-2-eno[3,2-c]pyrazol-16,17-diol
Description
16β-Hydroxystanozolol-D3 is a high-purity reference standard produced and distributed by WITEGA Laboratorien Berlin-Adlershof GmbH. It supports LC-MS/MS and GC-MS quantification, calibration, method validation, and confirmatory analysis. Use it for traceable workflows in compliance-driven environments. Key applications include deuterated steroid metabolite studies, LC-MS/MS calibration standard tasks, anti-doping screening, metabolism study reagent work, and residue control analysis.
This reference standard enables confident identification and accurate quantification. Its deuterium labeling enhances selectivity and reduces interferences. Consequently, analysts can build stable calibration curves and verify recovery with precision. The material fits seamlessly into routine methods and advanced research. Moreover, it provides dependable performance across varied matrices and concentration ranges.
- Robust results in LC-MS/MS and GC-MS workflows.
- Traceability to support audits and regulatory submissions.
- Reliable calibration and verification across dynamic ranges.
- Optimized for multi-residue method development.
Typical use cases include regulated laboratories, pharmaceutical research, residue control programs, and metabolism studies. It also suits anti-doping investigations that require confirmatory analysis. Therefore, the reference standard helps reduce uncertainty and strengthens data integrity. Furthermore, it streamlines method transfer and routine system suitability checks.
- Regulated laboratories seeking reproducible calibration and validation.
- Pharmaceutical research exploring steroid metabolism pathways.
- Residue control laboratories building rugged multi-residue methods.
- Confirmatory analysis that demands specificity and accuracy.
The 16β-Hydroxystanozolol-D3 reference standard from WITEGA Laboratorien Berlin-Adlershof GmbH supports traceable reporting and defensible results. Use it to refine calibration models, validate recovery, and confirm findings. With targeted transitions, it improves selectivity and sensitivity. In sum, 16β-Hydroxystanozolol-D3 strengthens reproducibility and confidence in quantitative measurements.
Safety Data Sheet
You can download your Safety Data Sheet for ST038
For other languages please contact us: witega@witega.de
Additional information
| Chemical name | (5α,16β)-17-Methyl-D3-1’H-androstano[3,2-c]pyrazol-16,17-diol(5α,16β)-17-Methyl-D3-2’H-androst-2-eno[3,2-c]pyrazol-16,17-diol |
|---|---|
| Molecular Formula | C21H29D3N2O2 |
| Molecular Weight | 347.51 g/mol |
| Isotopic purity | 99.8 atom% D (MS) |
| HPLC purity | > 99.0 % |
| Overall purity | > 99.0 % (HPLC) |
| Product Format | Neat |
| Delivery time | In stock |
| shelf life | 24 months |
| Storage | refrigerator, 2-8°C |
| Country of Origin | Germany |
| Product No. | ST038 |
| CAS Reg. No. | not available |
| Alternate CAS Reg. No. | 439791-84-1; 125590-76-3 (unlabelled compound) |
| Offer | 2.5 mg, 5 mg, 10 mg |