| Product No. | LA029 |
|---|---|
| CAS Reg. No. | 27164-46-1 |
| Alternate CAS Reg. No. | - |
| Offer | 100 mg |
27164-46-1
- Documentation
- Details
Chemical name
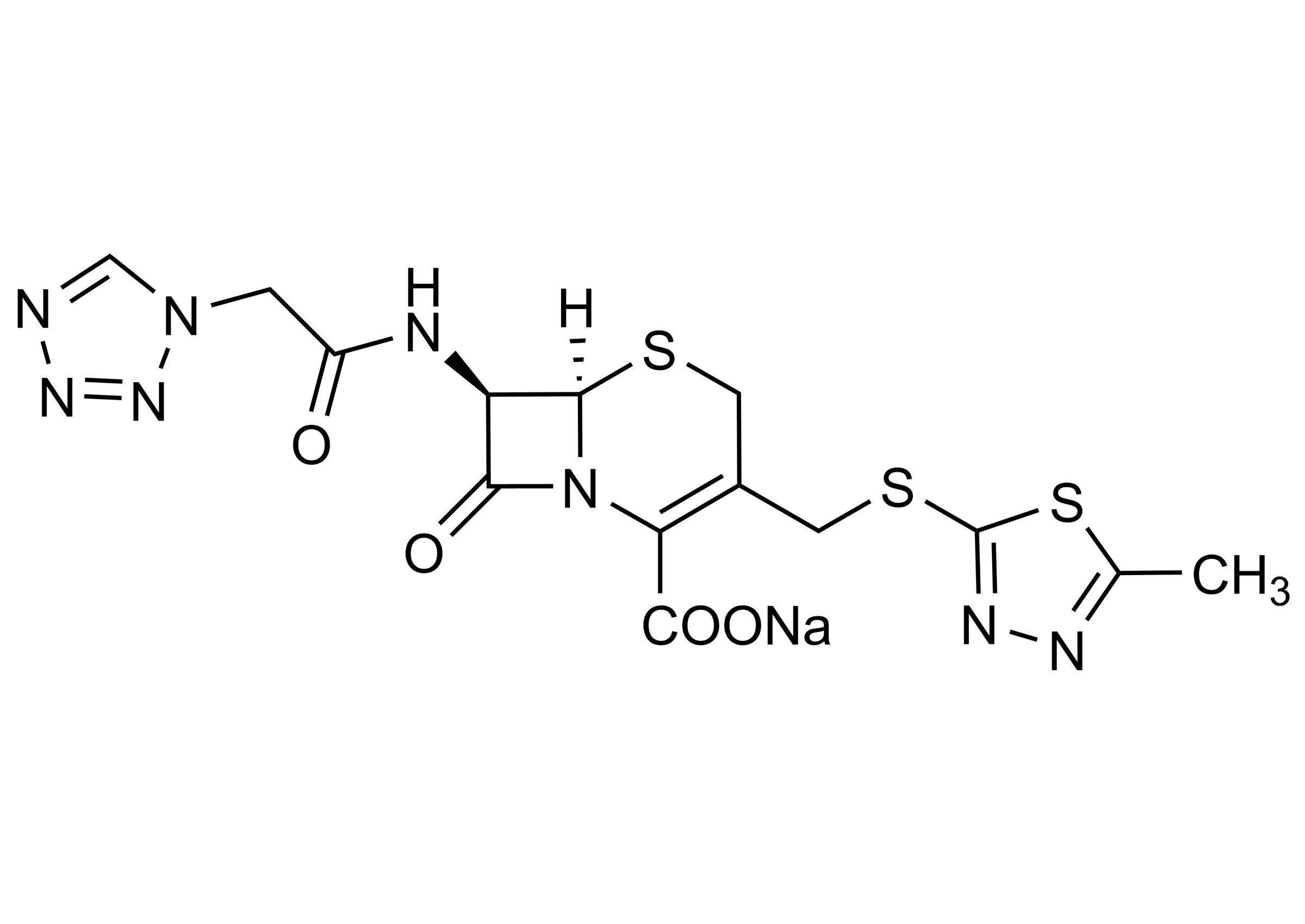
(6R,7R)-3-[[(5-Methyl-1,3,4-thiadiazol-2-yl)thio]methyl]-8-oxo-7-[[2-(1H-tetrazol-1-yl)acetyl]amino]-5-thia-1-azabicyclo[4.2.0]oct-2-ene-2-carboxylic acid sodium salt
Description
Cefazolin sodium salt (CAS 27164-46-1) is a high-purity reference standard produced and distributed by WITEGA Laboratorien Berlin-Adlershof GmbH. As a cephalosporin analytical standard and beta lactam reference material, it supports LC-MS/MS and GC-MS workflows from the start. This traceable calibration material enables robust quantification, clear identification, and reliable recovery assessment. Moreover, it helps maintain data integrity across batches. Developed for professional laboratories, it comes with comprehensive documentation for transparency and traceability.
Use this reference standard to strengthen your analytical process from calibration to confirmation. It performs in LC-MS/MS or GC-MS for quantitative assays, screening, and follow-up checks. Furthermore, it supports method validation tasks and routine quality control.
- LC-MS/MS quantification and calibration with full traceability.
- GC-MS confirmatory analysis and system suitability checks.
- Method validation covering accuracy, precision, linearity, and LOQ/LOD.
- Quality control for batch release and trend monitoring.
Designed for demanding environments, it aligns with regulatory expectations and good laboratory practice. Typical applications include:
- Regulated laboratories operating under GLP, GMP, or ISO 17025.
- Pharmaceutical research for development and stability studies.
- Residue control in clinical, environmental, or food matrices.
- Metabolism studies and biotransformation profiling.
- Multi-residue method development and robustness testing.
The Cefazolin sodium salt (CAS 27164-46-1) reference standard supports calibration curve preparation, matrix-matched verification, and confirmatory analysis. Every lot is released under stringent specifications to ensure consistency. For current documentation, including CoA and safety information, please contact WITEGA Laboratorien Berlin-Adlershof GmbH. Choose this reference standard to achieve repeatable results, defend your data, and meet audit expectations with confidence.
Safety Data Sheet
You can download your Safety Data Sheet for LA029
For other languages please contact us: witega@witega.de
Additional information
| Chemical name | (6R,7R)-3-[[(5-Methyl-1,3,4-thiadiazol-2-yl)thio]methyl]-8-oxo-7-[[2-(1H-tetrazol-1-yl)acetyl]amino]-5-thia-1-azabicyclo[4.2.0]oct-2-ene-2-carboxylic acid sodium salt |
|---|---|
| Molecular Formula | C14H14N8NaO4S3 |
| Molecular Weight | 476.49 g/mol |
| Isotopic purity | - |
| HPLC purity | > 99.0 % |
| Overall purity | > 97.0 % |
| Product Format | Neat |
| Delivery time | In stock |
| shelf life | 24 months |
| Storage | -20°C |
| Country of Origin | Germany |
| Product No. | LA029 |
| CAS Reg. No. | 27164-46-1 |
| Alternate CAS Reg. No. | – |
| Offer | 100 mg |