| Product No. | PS347 |
|---|---|
| CAS Reg. No. | 203645-57-2 |
| Alternate CAS Reg. No. | 959-98-8 (unlabelled compound) |
| Offer | 10 mg |
203645-57-2
- Documentation
- Details
Chemical name
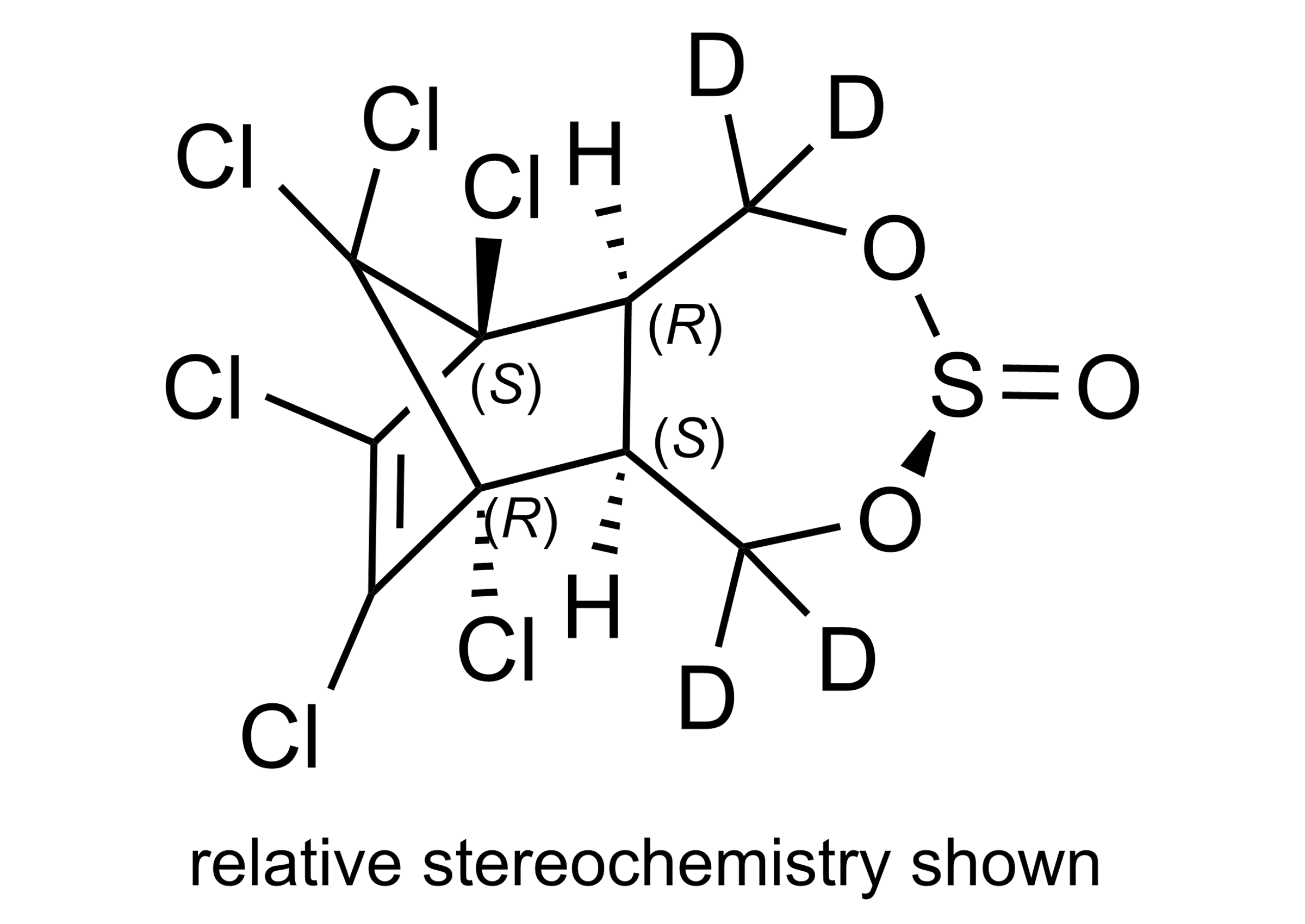
(3α,5aβ,6α,9α,9aβ)-6,7,8,9,10,10-Hexachloro-1,5,5a,6,9,9a-hexahydro-6,9-methano-2,4,3-benzodioxathiepin-1,1,5,5-D4-3-oxide, (mixture of diastereomers)
Description
alphaα-Endosulfan-D4 (CAS 203645-57-2) is a deuterated reference standard produced and distributed by WITEGA Laboratorien Berlin-Adlershof GmbH. This stable isotope labeled pesticide standard supports LC-MS/MS and GC-MS workflows for accurate quantification. It provides robust traceability for calibration, method validation, and confirmatory analysis across diverse and complex matrices.
Developed for quantitative residue testing, this reference standard helps you build reliable calibration curves and improve detection limits. It is suitable for routine monitoring, screening, and targeted confirmation. With consistent isotopic composition and high chemical purity, the material ensures reproducible response factors and precise analyte-to-analog matching. As a result, laboratories can streamline data review and reduce revalidation efforts.
Use this reference standard in regulated laboratories, pharmaceutical research, residue control programs, and metabolism studies. It also supports multi-residue method development, from optimization through full validation. Use it to verify recovery, evaluate matrix effects, and strengthen uncertainty budgets. Furthermore, its traceable preparation supports comparability between instruments, batches, and sites, improving long-term quality management.
- LC-MS/MS and GC-MS quantification with stable response.
- Calibration, instrument performance checks, and system suitability.
- Method validation: linearity, accuracy, precision, selectivity, and robustness.
- Confirmatory analysis for defensible reporting and audit readiness.
- Traceability that supports quality control and harmonized workflows.
- High purity and consistent isotopic labeling for precise quantification.
- Lot-specific information and documentation for transparent traceability.
- Optimized handling guidance to maintain integrity and stability.
- Suitable for matrix-matched calibration and ruggedness testing.
Choose this reference standard from WITEGA Laboratorien Berlin-Adlershof GmbH to enhance reliability in quantitative analysis, improve comparability between runs, and support compliant reporting.
Safety Data Sheet
You can download your Safety Data Sheet for PS347
For other languages please contact us: witega@witega.de
Additional information
| Chemical name | (3α,5aβ,6α,9α,9aβ)-6,7,8,9,10,10-Hexachloro-1,5,5a,6,9,9a-hexahydro-6,9-methano-2,4,3-benzodioxathiepin-1,1,5,5-D4-3-oxide, (mixture of diastereomers) |
|---|---|
| Molecular Formula | C9H2Cl6D4O3S |
| Molecular Weight | 410.95 g/mol |
| Isotopic purity | - |
| HPLC purity | > 99.0 % |
| Overall purity | > 99.0 % (HPLC) |
| Product Format | Neat |
| Delivery time | In stock |
| shelf life | 24 months |
| Storage | refrigerator, 2-8°C |
| Country of Origin | Germany |
| Product No. | PS347 |
| CAS Reg. No. | 203645-57-2 |
| Alternate CAS Reg. No. | 959-98-8 (unlabelled compound) |
| Offer | 10 mg |